Oculoplastic Surgery Unit. Fuenlabrada
Hospital. Madrid.
1 Graduate in Medicine and Surgery.
E-mail: ignaciogenol@gmail.com
2 Ph.D. in Medicine and Surgery.
INTRODUCTION
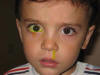
Congenital nasolacrimal duct obstruction (CNLDO) is a frequent entity in the pediatric age. It may affect 20-30% of newborns (1,2) [although only 1-6% exhibit symptomatic obstructions (3)], and affects 11% of pre-term births (4). Even though in most cases the epiphora is not an important problem, it does generate anxiety for the infant’s parents, above all when associated to secretion and/or repetition conjunctivitis or, in extreme cases, to acute dacryocistitis.
Although the management of CNLDO in patients under two years of age seems to enjoy some consensus, in older patients it is highly controversial (5). The objective of this paper is to review the therapeutic options and describe the advantages and drawbacks of each. Thereafter, therapeutic guide proposal for CNLDO is presented on the basis of the patient age, the clinical expressions of the condition and the experience of the authors, with the intention of assisting the ophthalmologists in their work.
As the most frequent cause of CNLDO (fig. 1) is the imperforation of Hasner’s valve in the outlet of the nasolacrimal duct in the inferior meatus, the majority of patients with drainage obstruction are empirically treated without requesting image tests or other confirmation procedures. These tests should be carried out if we suspect the presence of dacryocele or the patient associated craneo-facial alterations. It is not recommendable to perform dacryocystographies in pediatric age, with dacryscintigraphies being preferred for highly selected cases.
THERAPEUTIC OPTIONS: CONSERVATIVE TREATMENT
1. Observation
There are many papers describing a spontaneous resolution of the epiphora in up to 96% of CNLDO patients during the first year of life (6,7).
2. Lacrimal sac massage
The most widely used technique was originally described by Crigler (8) and consists in placing a finger on the internal edge of the infant’s eye to obstruct the common canalicule, preventing reflux when pressing the sac. Subsequently, exerting pressure on the sac, the finger is moved downwards, thus producing an increase in the hydrostatic pressure which is transmitted to the inferior portion of the nasolacrimal duct, causing the rupture of the obstructions located therein.
The effectiveness of the sac massage has been demonstrated by some authors and ranges between 85% and 95% (9-11), although there are no prospective studies comparing observation with said massage, so it cannot be concluded that the lacrimal area massage improves the percentage of CNLDO resolutions vis-à-vis the patients who are simply in observation. According to said authors, the possibilities of success of this massage seem to diminish when the patient is over 9 months of age.
THERAPEUTIC OPTIONS: SURGICAL TREATMENTS
1. Probes and irrigation
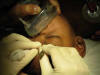
There is a broad consensus about the use of probes as the next step when the CNLDO failed to respond to conservative treatment (12,13). However, the best time for performing the probing or the type of anesthetic induction are not determined. The practice of this technique (fig. 2) is broadly disseminated among ophthalmologists, with an abundance of literature on the technique (14).
The main complication of the probe is the creation of false pathways (frequent in complex CNLDOs or in probes without anesthetic relaxation of the patient) that lead to failure of the procedure (5).
The percentage of success for the probe procedure is of approximately 85% (3,9,12) in infants under one year. This percentage diminishes as of this age. However, there is controversy in the percentage of success according to the patient age. While some authors obtain similar success rates (around 85%) in children over 1 year when compared to children under said age (15,16), others do not achieve the same results (17,18) and obtain a success rate of 89% in children under 2 years and of 72% in patients above said age.
The probe and irrigation procedure in patients younger or older than 12 months has its advantages as well as drawbacks. On the one hand, early probing exhibits the advantage that the resolution of the epiphora and conjunctival secretions improves considerable the likely irritative dermatitis of the patient eyelids and face. It also alleviate the anxiety of the parents. On the other, depending on the size and activity of the infant, the procedure could be carried out without requiring anesthesia utilizing a sheet in the practice for immobilization. From the cost-effectiveness viewpoint, it’s the most economic option (19) although it has the important drawback of submitting the child to a psychologically traumatic experience when anesthesia is not used. Considering the risk of creating false pathways due to sudden head movements, in our unit we prefer to utilize sedation for probings in the operating theatre but without managing the air pathways. However, when performing probings in infants under 1 year of age we assume the loss of the possibility that the natural history of the conditions will resolve the condition. In addition some authors have proposed that maintaining a conservative approach in the first year of life does not translate into an increase of epiphora cases in later age groups (20).
Different studies have surveyed the attitude of parents to early probing. According to Stager (21), 95% of parents estimated that the procedure had been much simpler and less invasive than they expected, 86% were satisfied with the fact that the procedure had been carried out in the practice and 81% preferred a new probing instead of waiting for spontaneous resolution. Recently, a validated quality of life questionnaire was presented in the United States, which should be useful to obtain more precise results in future clinical studies on CNLDO.
One of the main problems we could face in a late probing is that the very delay could give rise to acute infections with the ensuing theoretical risk of prolonged inflammation of the lacrimal mucosa and secondary fibrosis of the lacrimal pathway.
It must be emphasized that when the symptoms persist in children older than 1 year, this age group exhibits more complex obstructions (23), which are the most probable cause of probing failure (24). In a similar study, Kassoff (25) verified that the late probing with general anesthesia was the highest cost strategy for the hospital.
When the first probing fails, the most frequent alternative is to repeat it because, as an isolated procedure, it continues to have a high success percentage [approximately 85% (26,27)], particularly in infants under 24 months. At this stage and depending on the age of the child and the symptoms, we could consider associating a secondary procedure to the probing such as bicanalicular intubation, high pressure irrigation, dacryoplasty with balloon or nasal exploration with intra-operative endoscopy.
2. Intubation with silicone tubes (fig. 3)
The success percentage ranges between 77% and 100% according to
different studies (28-31). In addition, this procedure preserves the anatomy of
the lacrimal pathway. For this reason it is recommended to apply this technique,
always under general anesthesia, before considering other more invasive
procedures. The majority of ophthalmologists maintain intubation between 2 and 5
months (26,32,33), although good results were obtained with early removal after
6 weeks (34). The standard bicanalicular intubation procedure (BCNI) was
perfected by Crawford (35) in 1989, with the main potential complications being
the appearance of scar areas in the inferior meatus, the perforation or
laceration of the canaliculus and the extrusion of the silicone tube. An option
is mono-canalicular intubation through the upper canaliculus which, although
having the advantage of being easier removal and a similar success rate (90% in
children over 24 months) (36), it could be easily extruded (43%) (37). A
procedure recently described by Mauffray (38) consists in a dual bicanalicular
intubation as a step prior to DCR, with 80% success.
Some experts such as Geoffrey Rose (London) or Timothy J Sullivan (Brisbane) do
not intubate patients for a number of reasons (39):

Fig. 3. Silicone tubes for canalicular intubation.
-
First, there is no convincing evidence that intubation is more efficient than isolated probings.
-
Second: silicone tubes can produce inflammatory reactions leading to lacrimal pathway (particularly canalicular) stenosis, a considerably difficult condition to treat.
-
Third: having to remove the intubation involves an added trauma in the lacrimal pathway (with the risk of damaging it), particularly when removed via the canalicular system due to the knot.
3. High pressure irrigation (HPI) (40)
HPI is an alternative method for CNLDO in children under 18 months. As it is a recent development, there is hardly any experience in its use or conclusive results. Saline is injected through the lower lacrimal point under topical anesthesia with a 10 ml syringe and a 21G cannula. According to the author, the percentage of success is 80% at the first attempt and 100% if a second HPI is required. No complications were described in the 39-case CNLDO series. From our viewpoint, this procedure could give rise to lung problems due to inadvertent aspiration of the irrigated fluid.
4. Balloon dacryoplasty (figs. 4 and 5)
This procedure was described by Becker in 1996 (41) and has widely proved its usefulness (42-46) with a success percentage between 75 and 95%.

Fig. 4. Dacryoplasty catheter. Balloon dilated with saline (left) and undilated
(right).
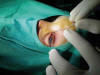
Fig. 5. Dacryoplasty via upper canaliculum, showing the probe marks.
The procedure is indicated for patients with 1 or 2 failed probings, particularly those over 30 months of age. The failure rate of probings in this age group is very high and the results are highly encouraging. Recently, in a well-designed study, Alañón (47) stated that balloon dacryoplasty is useful in 83% of patients and that his results are not affected by the type of obstruction or the age of patients, although it is preferably reserved for cases in which two previous probings have failed, associating in all cases monocanalicular intubation (48).
5. Nasal endoscopy and fracture of the inferior turbinate
Probe failures are usually due to (table 1):
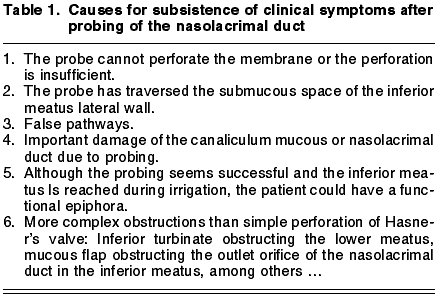
1. The probe enters the submucous creating false pathways without perforating the obstruction.
2. The presence of an inferior turbinate which anatomically prevents free access of the probe into the lower meatus.
3. The presence of nasal mucous flaps covering the ostium in the lower meatus, acting as a valve.
For these reasons it is highly recommendable to carry out a nasal endoscopy when the patient does not respond to the first therapeutic measures to visualize in detail the lower meatus anatomy. The inferior turbinate is fractured when we want to displace it from the ostium of the nasolacrimal duct to avoid mechanical obstructions of the lacrimal drainage, pushing the turbinate medially and upwards with a Freer elevator (49). Classically it has been said that in patients with a first probing failure, 88% of success is obtained when combining a second probing with an inferior turbinate fracture (50), but the fact is the presence of a large and poorly oriented turbinate is not frequently the cause of persistent epiphora. Accordingly, its displacement is not necessary in the majority of cases (51).
In our view, endoscopy is a useful measure when the second probing fails or the clinical history leads us to suspect a pathology in the nasal fossa.
6. Dacryocystorhinostomy (DCR)
This procedure is reserved for the cases in which all the above measures have failed and the CNLDO is located in the inferior portion of the lacrimal pathway, that is intrasac in the junction of the sac with the duct or in the duct itself (52). Other indications for DCR in pediatric age are canalicular atresia with or without lacrimal point agenesia, and in acquired pathologies of the canalicula (53). The use of silicone tubes for intubation of patients is a matter of debate. Some authors do not find differences in the percentage of success between intubated cases vis-à-vis without intubation (54). Other authors even consider that intubation diminishes the surgery success rate (55). In our experience, we prefer to use silicone tubes to prevent the osteotomy closure because young children have a higher scarring potential than adults. This procedure is performed under general anesthesia and the approach can be endoscopic or external. The success percentage of external DCR is similar to that obtained in adults (88-93%) (56). As regards endoscopic DCR in children, there are few published series and small numbers thereof (57,58), although with very good results (92-95%). It is true that there is a belief that the success percentage using the endoscopic approach is lower because the upper third of the sac cannot be surgically manipulated through the nose, but the fact is that there aren’t any conclusive studies because the inclusion criteria of the various studies render comparisons between results subjective. We always expect the patients to be over 24 months (there is no optimum age for performing DCR), even though the operations on children between 12 and 18 months (52) do not produce alterations in bone development. One final option is transcanalicular laser DCR, which doesn’t seem to be an effective method although to date the series remain small (59).
CONCLUSION AND ALGORITHM (Scheme 1)
Patients under 9 months: always apply conservative treatment [observation, massage as per Crigler’s technique (8)]. Only in exceptional cases, when the clinical expression is very florid and the parents’ anxiety is very high we would propose a first probing, warning in the informed consent about the above-mentioned disadvantages of intervening at such an early age. The only indication for probings in infants under 9 months of age is when the patient exhibits a congenital dacryocele (also known as neonatal acute dacryocistitis or congenital lacrimal sac mucocele).
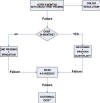
Schema 1. Proposed CNLDO algorithm.
* Determine if inferior turbinate fracture is required.
** Leave bicanalicular intubation for 3-6 months.
Patients over 9 months: always lacrimal pathway probing as first measure, under sedation without air pathways manipulation What to do if the first probing fails? We have four therapeutic options: 1) repeat probing, 2( silicone tube intubation, 3) dacryoplasty, 4) nasal endoscopy and interior turbinate fracture.
We never perform an HPI. According to the patient age and experience with the first probing, we utilize one technique or the other.
As the first step after the first probing failure, we always perform a second probing and mild irrigation under endoscopic control (determine whether the turbinate must be displaced and the irrigation fluid aspired). If the child is over 30 months, in addition to the probing we perform a balloon dacryoplasty.
If this first step fails (2nd probing without dacryoplasty), we perform bicanalicular intubation (if the patient is over 30 months, in the same surgical act we previously perform dacryoplasty), withdrawing the tubes after 6 weeks in surgery and irrigating.
If symptoms persist after these procedures, we perform an external DCR.
REFERENCES
-
Piest KL, Katowitz JA. Treatment of congenital nasolacrimal duct obstruction. Ophthalmol Clin North Am 1991; 4: 201-209.
-
Robb RMM. Congenital nasolacrimal duct obstruction.