REQUESTED COMMUNICATION
Treatment of neovascular glaucoma. The role of antiangiogenic drugs
MARTÍN GIRAL E1, PERUCHO MARTÍNEZ S2, FERNÁNDEZ ESCÁMEZ CS1, TOLEDANO FERNÁNDEZ N2
University Hospital
of Fuenlabrada. Ophthalmology Service. Madrid, Spain.
1
Graduate in Medicine.
2
Ph.D. in Medicine.
ABSTRACT
Neovascular glaucoma (NVG) designates a type of relatively frequent glaucoma which has a devastating effect on the visual potential and which arises secondary to the proliferation of neovessels over the iris and the chamber angle.
The main stimulus that promotes the development of NVG is severe retinal hypoxia, which can appear associated to severe diabetic retinopathy, vascular occlusions, ocular ischemia syndrome, cup-shaped cell disease or long-term retina detachment.
The appearance of new antiangiogenic drugs has caused a revolution of sorts in the treatment of this pathology, although many questions remain to be answered.
The objective of this revision is to update the knowledge available to date on the use of new antiangiogenic drugs in neovascular glaucoma.
Key words: Antiangiogenics, neovascular glaucoma.
NEOVASCULAR GLAUCOMA (NVG). DEFINITION AND MECHANISM
NVG is a type of glaucoma due to secondary angle closure without pupil obstruction. It is a clinical condition derived from a retinal ischemia or intra-ocular inflammation which can have various origins (table 1). The most frequent causes are diabetes mellitus, central retinal vein occlusion and ocular ischemic syndrome.
The disease is characterized by the proliferation of fine vessels over the surface of the iris and trabecular mesh which take hold on a transparent fibrous membrane which, when it contracts, produces synechiae which cause the secondary angular closure. Even though this closure is generally progressive, at the clinical level NVG usually expresses as acute or sub-acute glaucoma with pain, photophobia, epiphora and increased intra-ocular pressure (IOP), as well as diminished visual acuity (VA).
At the clinical level we can find the following stages (1):
-
Rubeosis stage (pre-glaucoma stage), characterized by normal IOP values. A thin rubeosis can be observed. The angle could be neovascularized or not but it is always open.
-
Open angle glaucoma stage, where we find a more flourished rubeosis with inflammatory reaction in the anterior chamber. Neovessels can be found in the angle and the patient exhibits a progressive increase of IOP. In this stage, hyphema could appear with debut of acute glaucoma.
-
Closed angle glaucoma stage. Synechial closure of the angle, partial or complete, usually with the presence of uveal ectropion. In this stage, glaucoma is usually severe with significant IOP increases. Surgical treatment is usually necessary.
Differential diagnostic (1,2)
In the open angle stage we must differentiate the condition from other acute glaucomas with neovessels in the iris:
-
Glaucoma associated to uveitis (Fuchs heterochromic uveitis).
-
Physiological vessels in stroma of clear iris.
In the closed angle phase we must differentiate it from other glaucomas due to angular closure with other etiologies:
-
Traumatism.
-
Iris-corneal-endothelial syndromes.
-
Phakogenic glaucoma.
-
Tumors, etc.
ANGIOGENESIS AND VASCULAR ENDOTHELIAL GROWTH FACTOR
Angiogenesis is regulated by several pro-angiogenic and anti-angiogenic factors. An imbalance of these factors brings about the formation of neovessels. Numerous papers have demonstrated that VEGF is a key element in the angiogenesis process. VEGF belongs to a family of proteins which includes VEGF-A, VEGF-B, VEGF-C, VEGF-D and PGF (placental growth factor). Its effect is regulated by three receptors with tyrosine-kinase activity: VEGFR-1, VEGFR-2 and VEGF-3. VEGF-A and its isoforms are considered to be the main mediators of pathological neovascularization. For this reason, the classical VEGF term alludes to VEGF-A. There are five isoforms of VEGF-A generated through the alternative splicing of the same messenger RNA molecule transcribed from a single gene. The most studied isoforms are VEGF121, VEGF165 and VEGF189. The nomenclature refers to the number of aminoacids comprising the mature molecule. For example, VEGF165 comprises 165 aminoacids and typically appears as a homodimer.
VEGF induces the proliferation of endothelial cells, promotes cell migration and inhibits apoptosis. In vivo, VEGF induces angiogenesis and increases vascular patency, playing a central role in the regulation of vasculogenesis. In addition, VEGF is a powerful mitogene, specific for vascular endothelium cells. Of all the molecules released by ischemic activity, VEGF seems to be the only one endowed with mitotic activity over endothelial cells. The association between the VEGF levels and neovascularization of the cornea, iris, retina and choroids has been verified in experimental models. When VEGF is selectively inhibited in animal models, a suppression of neovessel growth in tissues can be observed (3). The correlation in humans, involving VEGF and its receptors in neovascular disease, has been demonstrated in several studies involving patients with diabetic retinopathy, retinal vein occlusion, neovascularization of the iris, prematurity retinopathy and exudative age-related macular degeneration. The data obtained from all patients involved VEGF in the pathological vascular growth process (4).
TREATMENT OF NVG (fig. 1)
Neovascular glaucoma treatment is based on three pillars:
a) Treatment of the etiopathogenic
factor.
b) Immediate treatment of acute IOP increases.
c) Treatment of residual glaucoma.
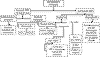
Fig. 1: NVG management algorithm.
A) Treatment of the etiopathogenic factor:
1. Diabetic retinopathy: panphotocoagulation (PFC) is indicated in cases of proliferative diabetic retinopathy or severe non-proliferative diabetic retinopathy with added risk factors such as neovascularization in the other eye, poor metabolic control, poor compliance of the base disease treatment or cataracts in evolution which will impede an adequate visualization of the ocular fundus (5). The regression of neovessels after a complete PFC occurs between four and six weeks after the intervention. In cases exhibiting opacity (severe and persistent hemovitreous or mature cataracts) which prevent adequate visualization and execution of the procedure, vitreo-retinal surgery and endophotocoagulation should be prescribed.
2. Central retinal vein thrombosis:
PFC is indicated only when neovascularization already exists, and not in a prophylactic manner (6). The regression of neovessels is achieved in 4-8 weeks.
3. Ocular ischemia syndrome: in this case, PFC is controversial because the ischemia originates in the uvea instead of the retina itself (7). Ocular hypotensors can help reduce the intraocular perfusion pressure.
4. Rest of pathologies: For tumors, Uveitis, vasculitis, traumatism, etc., apply the corresponding etiologic treatment.
B) Acute high IOP control: The recommendations of the European Glaucoma Society (8) are:
1. Corticoids: Prednisolone acetate 1% every 4/6 hours
2. Cyclopegics: 1% Atropine every 8/12 hours.
3. Hypotensors: betablockers, alpha agonists and topical or systemic carbon anhydrase inhibitors. The use of prostaglandin analogs is not advised due to their possible pro-inflammatory effect. The same applies to pylocarpine due to its myotic effect.
C) Treatment of residual glaucoma: up to now, neovascular glaucoma has been considered as a surgical pathology (9). Possible procedures include:
1. Conventional trabeculectomy with or without anti-mitotics: when the base disease is controlled, without complete synechial closure and inactive neovascular process.
2. Drainage devices: with uncontrolled neovascular activity, synechial closure in evolution and high recurrence risk, it is recommended to place the tube in sulcus and associated phakoemulsification (10).
3. Cyclodestructive procedures: recommended as second line of treatment for controlling pain in eyes with absolute glaucoma.
ROLE OF ANTIANGIOGENICS
Antiangiogenic drugs (Anti-VEGF) bond to the endothelial growth factor (VEGF) and prevent their proliferative action on vessels. Bevacizumab (Avastin R, Genetech Inc., San Francisco, CA, USA) and Ranibizumab (Lucentis R, Genetech Inc., San Francisco, CA, USA) are anti-VEGF monoclonal antibodies differentiated by the molecular size. Ranibizumab is smaller as it lacks the part of the molecule that acts as carrier, which facilitates its penetration in the retina but can also diminish its mean life inside the vitreous cavity. In turn, Pegaptanib (Macugen R, Eyetech Pharmaceuticals Inc. NY, USA) is an aptamere that acts only against the VEGF-165 isoform which accounts for ocular neovascularization and is therefore more selective than the other mentioned drugs. However, at present the drug which is most researched for treating neovascular glaucoma is Bevacizumab.
The above drugs have brought about a small revolution in the treatment of neovascular glaucoma. It seems demonstrated that Anti-VEGFs induce the regression of neovessels in a few hours, control the synechial closure and reduce the associated congestion and inflammation (fig. 2). However, this does not exclude the necessity of ablative treatment of the ischemic retina or the need of filtrating surgery in the vast majority of cases, although it does allow executing said treatments with less urgency, in a regulated manner, with fewer complications and better end results.
As far as we know, at present only one controlled, randomized and masked clinical trial comprising cases and controls has been published (11). The author demonstrated that intravitreal Bevacizumab, administered in a dosage of 2.50 mg in three injections four weeks apart is efficient to control iridian neovascularization and IOP in NVG treatments. The author describes that the effect remains six months later, and that the treatment is always made as an adjuvant to surgery and laser treatment when required.
It seems clear that the antiangiogenic effect of these substances is temporary. It seems that the only way to sustain it in time is when carrying out an ablative treatment of the ischemic retina together with the injection of the substance. The literature refers that the antiangiogenic effect has persisted up to 12 months after three Bevacizumab injections (12). When the complete angular closure takes place, the prognosis of the disease becomes darker and the reaction to treatments more unpredictable. Some studies (13) have expressed the importance of carrying out the treatment with anti-VEGF as early as possible.
In what concerns dosage, there are two guidelines: 1.25 mg/ml or 2.50 mg/ml injections. One paper (14) compared the effect of intravitreal Bevacizumab at a dosage of 1.25 mg and 2.50 mg in a single injection prior to trabeculectomy without finding statistically significant differences. Most publications utilize the 1.25 mg guideline as it appears that doubling the dose does not provide added benefits.
As regards the administration location, it can be either the anterior chamber or the vitreous cavity. Published studies (15,16) have demonstrated good results with both locations. The vitreous cavity injection appears to have the theoretical advantage of delivering the drug close to the retina, its therapeutic target. However, the intra-chamber injection is accessible to all ophthalmologists due to its simplicity and safety. The immediacy of the antiangiogenic effect seems to be the same regardless of the administration location. Even so, we have not found controlled studies demonstrating the superiority of one location over another.
In what concerns the number of injections, no consensus has been identified. It does seem clear that in many cases a single dose is not enough. The fact of applying three injections at 4-week intervals could provide good results and give sufficient time to carry out laser or surgical treatment as necessary (11).
CONCLUSION
Antiangiogenics, particularly Bevacizumab, have become a tool of undeniable usefulness for managing the highly aggressive neovascular glaucoma which is difficult to manage and in most cases with poor prognosis. The simplicity of its administration, the speed of the antiangiogenic effect and the very low number of reported complications are arguments supporting its use as an adjuvant treatment for managing this pathology. However, adequately controlled clinical trials are necessary to confirm these findings with more substantial scientific evidence.
REFERENCES
-
Shields MB. Textbook of Glaucoma. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2005.
-
Stamper RL, Lieberman MF, Drake MV, eds. Becker – Shaffer´s Diagnosis and Therapy of the Glaucomas. 7th ed. St Louis: Mosby; 1999.
-
Adamis AP, Shima DT. The role of vascular endothelial growth factor in ocular health and disease. Retina 2005 Feb-Mar; 25(2): 111-118.
-
Ciulla TA, Rosenfeld PJ. Anti-vascular endothelial growth factor therapy for neovascular ocular diseases other than age-related macular degeneration. Curr. Opin. Ophthalmol. 2009 May; 20(3): 166-174.
-
Pareja-Rios et al., Guías de práctica clínica de la SERV: Manejo de las complicaciones oculares de la diabetes. Retinopatía diabética y edema macular. Arch Soc Esp Oftalmol [online]. 2009, vol. 84, n.9, pp. 429-450.
-
Gomez-Ulla et al. Manejo de las Oclusiones Venosas de la Retina. Guías de Práctica Clínica de la SERV.
-
Johnston ME, Gonder JR, Canny CL. Succesfull treatmente for the ocular ischemic síndrome with panretinal fotocoagulation and cerebrovascular surgery. Can J Ophthalmol. 1988; 23: 114-117.
-
Terminology and guidelines for glaucoma(3rd edition):Therapetic guides: secondary angle closure glaucoma.European Glaucoma Society 2009. Pp: 181.
-
Garcia Sanchez, Julian; Honrubia Lopez, Francisco. Actualizacion en el tratamiento del Glaucoma. Sociedad Española de Oftalmología. 2003.
-
Gutierrez Dia, Esperanza, Montero Rodriguez , Marta. Dispositivos de Drenaje para glaucoma. Ed Ergon 2002.
-
Yazdani S, Hendi K, Pakravan M, Mahdavi M, Yaseri M. Intravitreal bevacizumab for neovascular glaucoma: a randomized controlled trial. J Glaucoma Oct-nov 2009, vol18, nº 8: 632-637.
-
C. Costagliola et al. Intravitreal Bevacizumab injection for neovascular glaucoma: a survey of 23 cases throughout 12 months follow-up. Br J Clin Pharmacol/66: 5/667-673.
-
Intravitreal Bevacizumab to treat iris neovascularization and neovascular glaucoma secondary to Ischemic retinal dieases in 41 consecutive cases. Ophthalmology. 2008 Sep; 115(9): 1571-80.
-
Gupta V, Jha R, Rao A, Kong G, Sihota R The effect of different doses of intracameral bevacizumab on surgical outcomes of trabeculectomy for neovascular glaucoma. European Journal of Ophthalmology / Vol. 19 no. 3, 2009; pp. 435-441.
-
Susana Duch, MD, PhD, Oscar Buchacra, MD, Elena Milla, MD, PhD, David Andreu, MD, PhD, and Jesu´s Tellez, MD. Intracameral Bevacizumab (Avastin) for Neovascular Glaucoma.A Pilot Study in 6 Patients. J Glaucoma _ Volume 18, Number 2, February 2009; 140-143.
-
Intravitreal bevacizumab as an adjunct treatment for neovascular glaucoma. Hasanreisoglu M, Weinberger D, Mimouni K, Luski M, Bourla D, Kramer M, Robinson A, Axer-Siegel R. Eur J Ophthalmol. 2009 Jul-Aug; 19(4): 607-12.