REQUESTED COMMUNICATION
Serpiginous Choroiditis: activity indices
SALAZAR MÉNDEZ R1, CORDERO COMA M2
León University Hospital Complex.
Ophthalmology Service.
1 Ophthalmology Resident Physician. Central University hospital of
Asturias.
2 Deputy Ophthalmology Physician. Eeón Hospital.
ABSTRACT
Introduction: Serpiginous choroiditis (SC) is classified in the group of white spot syndromes (WSS). It constitutes a bilateral posterior uveitis with a chronic and progressive course, alternating active and quiescent stages. Its diagnostic is mainly based on ophthalmoscopic exploration, although this is frequently not enough to determine the activity of the condition and thus establish the most adequate therapeutic approach. This article reviews SC and analyzes value of various factors (visual acuity, ocular fundus exploration, fluorescein and indocyanine green angiography, campimetry, autofluorescence and electrophysiological tests) for monitoring the severity of the injuries.
Conclusions: the visual prognosis of SC is decisively influenced by the tendency towards inflammatory process recurrence. These recurring inflammation episodes cause a painless but progressive loss of eyesight, and therefore it is essential to identify and treat them in an early and aggressive manner. In this management it is necessary to assess various activity indicators, the knowledge and application of which is crucial for the follow-up and decision-making for these patients.
Serpiginous choroidopathy is an infrequent and bilateral disorder characterized by chronic, progressive and recurring inflammation of unknown etiology which affects the complex made up by the retina pigment epithelium (RPE) and the choriocapillary. It was first described in 1932 by Junius, although a complete description was not made until the sixties (1). Since then it has been given different names, including peri-papillary choroidal sclerosis, peripapillary helicoid chorioretinal degeneration, geographic choroiditis, geographic choroidopathy, peripapillary helicoid geographic choroidopathy, among others (2).
It accounts for less than 5% of posterior uveitis (in some regions such as India the prevalence is much higher, reaching 19%). It expresses in young or middle-aged adults, with many papers describing greater prevalence in males but without familial or racial predisposition (although a recent publication described the greater frequency of HLA B7 in said group). Even though the literature describes cases associated to various systemic processes like Crohn’s disease, distony, celiac, sarcoidosis or panartheritis, a causal relationship was not established in any case (2,3).
The condition is characterized by the appearance of the regular yellowish lesions in the peripapillary and macular area with serpiginous or helicoidal progression, although at least 3 differentiated patterns have been described. These are:
-
Typical or papillary geographic pattern: this is the most frequent pattern accounting for 80% of cases. The condition debuts with subretinal creamy spots with poorly defined edges in the peripapillary region, progressing outwardly following an irregular serpiginous pattern. Said lesions resolve after 6-8 weeks and are replaced by an atrophy of the RPE/ choriocapillary complex. It is frequent to find lesions in various stages of evolution, with frequent recurrence thereof at the edges of previous scars. (2,4)
-
Serpiginous macular choroiditis: about 59% of cases begin at the macular level (5). There are no demographic or angiographic differences with typical SC other than its location and the poor visual prognosis, secondary to foveal involvement and high risk of choroidal neovascularization (CNV). It is frequently misdiagnosed as age related macular degeneration (ARMD), toxoplasmosis or various macular dystrophies. (2,4)
-
Atypical variants (ampiginous choroiditis or relentless placoid chorioretinitis): occasionally, the lesions appear in the periphery either isolated or in the context of a multifocal pattern. In addition, the literature describes cases initially diagnosed as acute posterior multifocal placoid pigment epitheliopathy (APMPPE), with subsequent progression to SC. Compared to the typical forms there is less foveal involvement and therefore an improved visual prognosis (2-4,6).
In what concerns the pathological anatomy, even though there are very few histopathological studies, they have demonstrated the existence of lymphocytic diffuse infiltration as well as atrophy of photoreceptors, EPR and choriocapillary, the latter being the most affected layer (7).
A broad range of pathogenic theories have been proposed but none has been confirmed to date, with SC perhaps being the common expression to a high diversity of systemic processes:
-
Self immune: this theory is based on its recent association with CMH (HLA B7), reactivity against antigene S and frequent hypocomple¬mentemia C3 detected in patients (2,8,9).
-
Infectious: historically, SC has been related with tuberculosis and herpes infections (1,2,10). The relationship between this process and tuberculosis has been recognized since the fifties due to the appearance of choroiditis simulating SC lesions (tuberculous serpiginous-like choroiditis or TB¬SLC), which debuts as a multifocal choroiditis tending to coalesce in ameboid pattern (8,9). These lesions exhibit a relentless course regardless of systemic immunosuppression and respond in a satisfactory and definitive manner to antituberculosis treatment (ATT). The exact mechanism causing this condition is unknown, although it seems to represents a hypersensitivity reaction immunomediated by the tuberculosis bacillum (11,12). Recently, attempts have been made to establish the main clinical differences between typical SC and TB-SLC. Thus, TB-SLC usually courses with more intense vitritis and multifocal lesions distributed in the posterior pole as well as the periphery, with variable pigmentation (fig. 1), in contrast with typical SC where vitritis is minimal or absent and the lesions are circumscribed to the peripapillary and macular area (13). Latent TB has to be discarded as the first cause of SC due to its therapeutic and prognostic implications, preferably by combining the results of intradermal reaction and QuantiFERON TB-Gold (14). If the result is positive, it is necessary to establish an ATT treatment which will heal in the case of TB-SLC and required before considering immunosuppressive treatments for SC due to the high risk of disseminated TB.
-
Vascular: in some patients, SC can coexist with vasculopathies or coagulation disorders (increased von Willebrand factor VIII), as well as being accompanied in its expression by periphlebitis and vascular obstructions or sharing clinical appearance with the occlusion of ciliar retinal vessels (2,8,15).
-
Degenerative: based on its chronic and progressive course, the age of onset and eventual association with degenerative disorders (2).
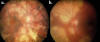
Fig. 1: Ophthalmoscopic appearance of (a) right eye
in a TB-SLC patient.
ACTIVITY PARAMETERS
The SC diagnostic is mainly based on funduscopic exploration (fig. 2). However, it is frequently difficult to determine the degree of activity of the lesions and therefore the therapeutic approach, based exclusively on the clinical appearance. For this reason, supplementary tests are particularly useful as they provide additional and highly valuable information for monitoring these patients. These tests include:

Fig. 2: Progressive course in patient with active
SC lesions.
– Visual Fields (VF): VA describes in a very limited manner the visual impact of chorioretinal diseases with macular repercussion such as SC (2). In these cases it is essential to quantify the macular function, for example by means of microperimetry, a non-invasive method that explores fixation and central VF defects. Recent studies have demonstrated conservation of fixation in over 60% of cases, even with extensive macular involvement. This seems to indicate that the existence of a minimal area of residual retinal sensitivity is useful to maintain fixation, even in SC cases with extended atrophy. In bilateral cases, stable and central fixation is detected in the eye with a better VA even though it exhibits the same atrophy lesions. A dense central scotoma is generally identified, surrounded by relative scotoma in the geographic lesions (corresponding to fugue areas in indocyanine green which cannot be seen with angiography). In addition, the activity of the process is usually correlated with the density of said Scotoma (16).
– Electrophysiological tests: the electroretinogram (ERG) and electro-oculogram (EOG) give normal results except cases with longer evolution and/or extensive lesions in the posterior pole (2).
– Fluorescein angiography (FAG): the findings and not pathognomonic. Atrophy areas are initially seen as hypofluorescent due to choriocapillary atrophy, with progressive hyper fluorescence in the edges and eventual scleral staining (fig. 3). The active lesions obstruct fluorescein and presents diffuse staining with progressive delayed loss (fig. 4). In the active phases it is frequently possible to identify sections of perilesional vasculitis (fig. 5) (2).
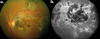
Fig. 3: (a) Ophthalmoscopic and (b) angiographic appearance of inactive macular
serpiginous lesion.
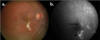
Fig. 4: (a) Ophthalmoscopic and (b) angiographic appearance of patient with
active lesion (arrow) and inactive lesion )arrow tip).
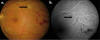
Fig. 5: (a) Ophthalmoscopic and (b) angiographic appearance of patient with
active SC and vasculitis (arrow).
– Indocianine green angiography (ICAG): in contrast with FAG, it allows the identification of different angiographic pattens on the basis of the evolution stage. Thus, during inactive choroiditis phases, it identifies the degree of choroidal atrophy and scarring, and is particularly useful for discarding or confirming the activity of a choroidal process. Whereas in FAG active lesions usually exhibit early obstruction with delayed staining which varies according to the degree of dysfunction of the blood-retina barrier, in ICAG the lesions appear hypofluorescent during the entire study due to the extensive destruction of the choriocapillary, even allowing in some cases the identification of lesions that cannot be seen with ophthalmoscopy. In addition, said hypo-fluorescence is more evident and better defined than that seen in FAG. ICAG allows the identification of 4 distinctive patterns based on the stage of evolution of the disease:
-
Subclinical stage (choroidal): Hypo- fluorescence with diffused edges, FAG and funduscopic exploration being normal because the RPE is not yet involved.
-
Active stage: The RPE is now involved and the lesions are visible through FAG and funduscopy. ICAG reveals early hypo-fluorescence with poorly defined edges which occasionally can be associated in particularly aggressive forms with delayed staining and dye diffusion (fig. 6).
-
Cicatricial stage: whereas FAG does not exhibit signs of activity at the level of the RPE, ICAG demonstrates the persistence of the inflammation at the choroidal level regardless of the cicatricial condition of the RPE, exhibiting a discrete persistent delayed loss.
-
Inactive stage: hypofluorescence with clearly defined edges, enhanced in delayed stain (fig. 7).
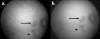
Fig. 6: Appearance in ICAG (a) early and (b) late, in the patient of figure 4,
with active (arrow) and inactive lesion (arrow tip).

Fig. 7: (a) Ophthalmoscopic, (b) angiographic and (c) ICAG appearance of
inactive lesion.
Accordingly, in comparison with FAG, ICAG allows for improved SC lesion staining, evidencing choroidal alterations which are not visible with FAG, as well as enhanced identification of the active lesions, which appear larger than the retinal lesions. Additional advantages of ICAG include the demonstration of choroidal staining and loss from vessels in half of patients, the identification of choroidal activity even in the absence of retinal activity, as well as the reduction in size of inactive areas after immunosuppressive treatment. In summary, it is a particularly useful tool for monitoring the disease as it provides a highly sensitive method to identify its stages, thus assisting in the therapeutic decision-making process (2,6,17,18).
– Autofluorescence: autofluorescence (AF) has been utilized to assess the condition of the RPE in various inflammatory, degenerative and neoplasic disorders. Its signal is primarily derived from the accumulation of lipofucsine at the RPE level, an indicator of structural and/or functional alteration. A controversial aspect is the usefulness of AF for differentiating the processes included within SMB, as well as its capacity to detect activity in said processes. In the case of SC, the hypoautoflouresence pattern matches the chorioretinal atrophy areas. In active cases, it is possible to see hyperautofluorescence areas matching the angiographic findings although in a highly evident manner, which makes hyperautofluorescence a highly sensitive indicator of activity in the edges of atrophic lesions. Likewise, it allows for the identification of CNV focused points as hyper-autofluorescence areas. On the other hand, for WSS a negative correlation has been generally demonstrated between the extension of the foveal hypoautofluorescent areas and visual acuity. Accordingly, FA constitutes a particularly useful non-invasive method for monitoring and following up SC patients. The changes on the different autofluorescence patterns in the course of SC have been recently demonstrated. In this manner, in very early stages a discrete hpoautofluorescence can be visualized accompanied by a tenuous hyper-autofluorescent halo in the active lesions, giving way after a few days to an openly hyper-autofluorescent pattern. After a few weeks and as the lesion resolves, a hypo-autofluorescent edge can be seen, followed by a granular pattern of the lesion and finally a well defined hypo-autofluorescence. On the other hand, treated lesions seem to exhibit lower hypo-autofluorescence, suggesting that immuno-suppression could reduce the damage to the RPE. Therefore, FA appears as a non-invasive method to differentiate various WSS conditions as well as for early detection of changes in the RPE and a determining factor for therapeutic decision-making (19,20).
– Optic Coherence Tomography (OCT): the tomographic characteristics of SC consist in hyper-reflectiveness isolated from the external retina, which seems to be correlated with histopathological findings which demonstrate an extensive loss of the RPE, with secondary destruction of the adjacent retina. Even though the ophthalmoscopic and angiographic findings can be similar in multifocal choroiditis and SC, OCT «en-face» allows to identify different morphological pattens, confirming the hypothesis about its pathogeny (choriocapillary disease with photoreceptor atrophy and variable choroidal damage). OCT allows not only for the identification of secondary CNV but also an improved knowledge about the location and extension of inflammatory chorioretinal lesions (21,22).
The differential diagnostic is considered mainly with other WSS, such as APMPPE or multifocal choroiditis, as well as with other disorders such as toxoplasmosis, tuberculosis, choroidal ischemia, sarcoidosis, syphilis or histo¬plasmosis, among others (2).
The most frequent complication having the highest visual impact on SC is CNV, which is described in 13-35% of cases and can constitute its debut. Probably, there is a relationship between the location of the lesions in the papillary-macular area and their special sensitivity to the action of vasoproliferative agents. These membranes can be successfully treated with laser photocoagulation, photodynamic therapy and intravitreal anti-VEGF. Differential diagnostic between subretinal neovascular membranes and the areas of activity is essential. Funduscopy could be difficult, particularly in the case of membranes with abundant subretinal liquid. In FAG, CNV appears as an early hyperfluorescence pattern which increases throughout the angiogram, accompanied by delayed loss, while the ICGA reveals a hot spot or more frequently a hyper¬fluorescent plate throughout the test. OCT is an essential diagnostic tool because, in the case of subretinal neovascular membranes, it confirms the presence of a subretinal hyper-reflective area accompanied by neurosensory detachment with accumulation of subretinal fluid (fig. 8). Additional complications include the occlusion of retinal venous branches, periphlebitis, pigment epithelium detachment, serous detachment, cystic macular edema, optic disc neovascularization, subretinal fibrosis and anterior uveitis (2).

Fig. 8: (a) Ophthalmoscopic, and (b) tomographic appearance of patient with SC
and CNV.
REFERENCES
-
Laartikainen L, Erkkila H. Serpiginous choroiditis. Br J Ophthalmol 1974; 58: 777-783.
-
Lim W-K, Buggage RR, Nussenblatt RB. Serpiginous choroiditis. Surv Ophthalmol 2005; 50(3): 231-244.
-
Gupta V, Agarwal A, Gupta A, Bambery P, Narang S. Clinical characteristics of serpiginous choroidopathy in North India. Am J Ophthalmol 2002; 134: 47-56.
-
Caspers L. Serpiginous choroiditis. In: Gupta A, Gupta V, Herbort C et al. Uvetis, text and imaging. New Delhi: Jaypee; 2009.
-
Munteanu G, Munteanu M, Zolog I. Serpiginous choroiditis: clinical study. Oftalmologia 2001; 52: 72-80.
-
Jones BE, Jampol LM, Yannuzzi LA, Tittl M, Johnson MW, Han DP et al. Relentless placoid chorioretinitis: a new entity or an unusual variant of serpiginous chorioretinitis? Arch Ophthalmol 2000; 118: 931-938.
-
Wu JS, Lewis H, Fine SL, Grover DA, Green WR. Clinicopathologic findings in a patient with serpiginous choroiditis and treated choroidal neovascularization. Retina 1989; 9: 292-301.
-
Quillen DA, Davis JB, Gottlieb JL, Blodi BA, Callanan DG, Chang TS et al. The white dot syndromes. Am J Ophthalmol 2004; 137: 538-550.
-
Rodriguez-García A. Serpiginous choroiditis. In: Foster CS, Vital AT. Diagnosis and treatment of uveitis. Philadelphia: WB Saunders; 2002.
-
Priya K, Madhavan HN, Reiser BJ, Biswas J, Saptagirish R, Narayana KM, Rao NA. Association of herpesviruses in the aqueous humor of patients with serpiginous choroiditis: a polymerase chain reaction-based study. Ocul Immunol Inflamm. 2002; 10: 253-61.
-
Gupta V, Gupta A, Rao NA. Intraocular tuberculosis – an update. Surv Ophthalmol 2007; 52: 561-587.
-
Erkkila H, Laatikainen L, Jokinen E. Immunological studies on serpiginous choroiditis. Graefes Arch Clin Exp Ophthalmol 1982; 219: 131-134.
-
Vasconcelos-Santos DV, Rao PK, Davies JB, Sohn EH, Rao Na. Clinical features of tuberculous serpiginouslike choroiditis in contrast to classic serpiginous choroiditis. Arch Ophthalmol 2010; 128: 853-858.
-
Mackensen F, Becker MD, Wiehler U. QuantiFERON TB-Gold – a new test strengthening long-suspected tuberculous involvement in serpiginous-like choroiditis. Am J Ophthalmol 2008; 146: 761-766.
-
King DG, Grizzard WS, Sever RJ, Espinoza L. Serpiginous choroiditis associated with elevated factor VIII-von Willebrand factor antigen. Retina 1990; 10: 97-101.
-
Pilotto E, Vujosevic S, Grgic VA, Sportiello P, Convento E, Secchi AG et al. Retinal function in patients with serpiginous choroiditis: a microperimetry study. Graefes Arch Clin Exp Ophthalmol 2010; 248: 1331-1337.
-
García-Saenz MC, Gili Manzanaro P, Bañuelos Bañuelos J, Villarejo Díaz-Maroto I, Arias Puente A. Valoración de enfermedades inflamatorias coriorretinianas con angiografía y verde indocianina. Arch Soc Esp Oftalmol 2003; 78(12): 675-683.
-
Giovannini A, Mariotti C, Ripa E, Scassellati-Sforzolini B. Indocyanine green angiographic findings in serpiginous choroidopathy. Br J Opthalmol 1996; 80: 536-540.
-
Yeh S, Forooghian F, Wong WT, Faia LJ, Cukras C, Lew JC et al. Fundus autofluorescence imaging of the White Dot Syndromes. Arch Ophthalmol 2010; 128(1): 46-56.
-
Cardillo Piccolino F, Grosso A, Savini E. Fundus autofluorescence in serpiginous choroiditis. Graefes Arch Clin Exp Ophthalmol 2009; 247: 179-185.
-
Gallagher MJ, Yilmaz T, Cervantes-Castañeda RA, Foster CS. The characteristic features of optical coherence tomography in posterior uveitis. Br J Opthalmol 2007; 91: 1680-1685.
-
van Velthoven MEJ, Ongkosuwito JV, Verbraak FD, Schlingemann RO, de Smet MD. Combined en-face optical coherence tomography and confocal ophthalmoscopy findings in active multifocal and serpiginous chorioretinitis. Am J Ophthalmol 2006; 141: 972-975.