REQUESTED COMMUNICATION
TNF-a and anti-TNF-a drugs in ophthalmology
RODRÍGUEZ-FEIJOÓ D1,3, PINAR SUEIRO S2,3, FONOLLOSA Á2,3
1
Ophthalmology Service. Galdakao-Usansolo Hospital, Vizcaya. Graduate in
Medicine.
2 Ophthalmology Service. Cruces Hospital, Vizcaya.
3 Experimental Ophthalmic – Biology Group (GOBE), Basque Country
University (UPV/EHU). Vizcaya.
INTRODUCTION
(Tumor Necrosis Factor Alpha, TNF-a) is a pro-inflammatory cytokine with multiple functions in the immune response. Since its initial discovery as a seric factor causing tumor necrosis, multiple functions have been found involving the regulation of inflammatory processes. This powerful immunological mediator also plays an important role in cell apoptosis and the defense against pathogen agents (1,2).
TNF-a is mainly synthesized in monocytes/macrophages and T-lymphocytes and, to a lesser extent, in neutrophiles, mastoid cells, endothelial cells, fibroblasts and glyal cells. TNF-a increases inflammation through a direct cytotoxic effect and also through indirect effects such as the production of other pro-inflammatory cytokines, arachidonic acid mediators, released of oxygen and nitrogen free radicals, metaloproteinases, chemokynes and antiangiogenic factors (3).
According to the typical pro-inflammatory model, failures in the TNF-a regulation at the site where the immunological response is occurring will activate innate cell immunity and chronic inflammatory response. The result of this is tissue damage. This has been demonstrated in experiments with transgenic mice that overexpress TNF-a in the joints. All of said mice developed inflammatory polyarthritis which responded to treatment with drugs utilized for human rheumatoid arthritis (RA) (4).
In clinical practice, the neutralization of TNF-a activity by means of drugs involves a deactivation of the pro-inflammatory cascade cytokins, diminished recruitment of inflammatory cells from the blood stream to the inflammation area, diminished angiogenesis capability regulated by vascular endotelial growth factor (VEGF), alterations in chemokynes and vascular patency. Anti-TNF-a drugs started to be applied in medicine in the late nineties to treat RA and Crohn’s disease (5,6). At present, these drugs are also prescribed for the treatment of juvenile idiopathic arthritis, psoriasic arthritis, ankylosing spondylitis, ulcerous cholitis and uveitis in ophthalmology.
TNF-a: STRUCTURE AND BIOLOGICAL FUNCTION
The TNF group comprises 19 cytokines with significant functions in inflammation and apoptosis (1). The TNF-a subtype is a 17 KD protein made up of 185 aminoacids. The biological activity of TNF-a is regulated through its TNFR1 receptors (solluble TNF-a receptor, expressed in the majority of cells) and TNFR2 (TNF-a receptor associated to membrane, present only in some cell types). Both receptors are transmembrane glycoproteins with similar extracellular domains. However, their intracellular domain is different and only TNFR1 has a «death domain» (a receptor located on the secular surface and that transmits apoptosis signals. These receptors can activate cellular apoptosis through a caspase cascade in a few seconds after union of the ligand) which is significant in the regulation of cellular apoptosis through TNF-a. For this reason, it was initially believed that TNFR1 regulates inflammatory and apoptosis responses while TNFR2 regulates cell growth and proliferation responses (7). At present, some studies (8,9) have also identified protecting functions regulated by TNFR1. The cell type and functional condition, the concentration and duration of exposure to TNF-a And the type of cytokins present we determine the activation of one type of response or another (scheme 1).
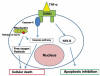
Scheme 1: The union of TNF-a
to TNFR1 is able to regulate cell death or survival.
TNFR1 and 2 receptors have been found in endothelial cells, in the iris, the ciliary body, the optic nerve and the vitreoretinal interface in mice (10). In humans, TNFR1 and 2 receptors have been found in the vitreous while in retina pigment epithelium cell cultures TNFR1 but not type 2 receptors have been found (11). TNF-a has been detected predominantly in the internal retina layers, which matches the glial cell distribution pattern in the retina, i.e., astrocytes and Müller cells. The presence of the TNFR1 receptor in retinal ganglion cells indicates their susceptibility to the cytotoxic effect of TNF-a. The functional basis of TNF-a in the retina resemble the operation of TNFa in the brain where, under normal situations, it acts as a neuronal modulator even though in ischemia it can have neurotoxic or neuroprotective effects (7).
TNF-a IN OCULAR DISEASES
TNF-a participates actively in the pathogenesis of inflammatory diseases, in the production of edema, neovascularization and neurodegenerative diseases at the ocular and intraocular level.
The relationship of TNF-a in uveitis has been demonstrated by means of 2 animal models of self immune uveitis and endotoxin-produced uveitis (12). In addition, polymorphisms have been found in the TNF-a Pene, which alters its expression causing an overproduction of TNF-a by leukocytes in Behçet’s disease (13).
There is scientific evidence suggested on the basis of modern animals and the study of human samples obtained during the vitreoretinal surgery on the role of TNF-a in the production of retinal neovascularization, proliferative vitreo-retinopathy (PVR) and retinal edema (14,15). It seems irrelevant to emphasize the role of TNF-a in PVR because it is an important cause of regmatogenous retina detachment surgery failure. The syntheses of TNF-a is augmented in eyes with PVR and normally regulates the expression of cell survival factors that protect retina pigment epithelium cells in PVR. Recently, a simple nucleotide polymorphism has been described in the TNF locus closely associated to PVR (16).
ANTI-TNF-a DRUGS
TNF-a drugs exhibit a foster reaction, excellent safety profile and very few but potentially very severe contraindications which must always be taken into account before beginning administration in order to prevent possibly severe complications such as poorly treated tuberculosis, repetition infections or recent severe infection treated with IV antibiotics, cancer in the past 5 years (without including skin basocellular cancer), pregnancy or lactation, severe heart disease or de-myelinizing disease.
The currently available anti TNF-a drugs are listed below (Table 1):
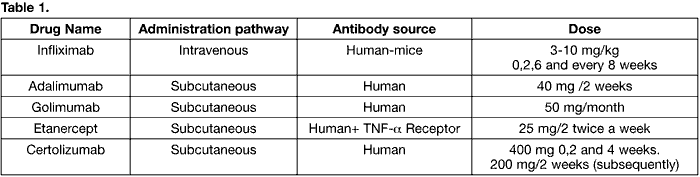
– AntiTNF-a monoclonal antibodies: Infliximab (Remicade®): chimeric monoclonal antibody (IgG) derived from a recombinant DNA, formed by a constant region of human origin and other mice variables. Infliximab has a mean life in saline of 10 days (17). It joins to and neutralizes the TNF-atransmembrane and solluble portion, interrupting the sequential activation cascade of the inflammatory pathways regulated by this cytokine. Administration is through intravenous perfusion in combination with an antihistaminic. The recommended dose is of 3-10 mg per kg of body weight. Generally, a dose is administered in weeks 0, 2 and 6 and every 8 weeks subsequently.
– Anti-TNF-a monoclonal antibodies: Adalimumab (Humira®): this is a biological drug that specifically blocks TNF-a. Its entirely human origin explains why Adalimumab induces lower immunoallergic reactions (formation of anti-adalimumab antibodies, allergic reactions) when compared to Infliximab. The usual dose of Adalimumab is of 40 mg every 2 weeks administered subcutaneously (18).
– Anti-TNF-a monoclonal antibodies: Golimumab (Simponi®): An entirely human monoclonal antibody, approved in 2009 by the FDA for treating RA and other self immune diseases. The administration pathway is subcutaneous in a dose of 50 mg once a month (19).
– Solluble TNF-a Receptor: Etanercept (Enbrel®): a molecule comprised by a recombinant dimeric protein linked to a portion of human TNFR2 and to the constant fraction of human IgG (20). It has a mean short life of about 4 days. Etanercept links to TNF-a. And renders it a biologically inactive, preventing it from linking to the receptors located in the membranes of the cells in charge of the inflammatory response. Administration is subcutaneous, twice a week in a dose of 25 mg in each injection.
– Anti-TNF-a Recombinant antibody fragment: Certolizumab pegol (Cimzia®): A human monoclonal antibody fragment expressed in Escherichia coli and conjungated with polyethyleneglycol (PEG). The dose of Certolizumab is of 400 mg (2 injections of 200 mg each in one day) at weeks 0, 2 and 4, followed by a maintenance dose of 200 mg every 2 weeks. The administration pathway is subcutaneous (21).
At present there is evidence derived of experimental studies in animals and trials in humans suggesting that TNF-a. Plays a specific role in ocular inflammation. TNF-a is high in serum and/or aqueous humor in patients with uveitis compared to controls (22), as well as in patients with vasculitis (23) and sympathetic ophthalmia (24). Due to these findings and to the limited clinical success or partial failure of steroids and immunosuppressants in controlling ocular inflammation in some patients, as well as the high rate of side effects of steroids and the clinical success that the anti-TNF-a drugs has demonstrated in other systemic inflammatory pathologists, ophthalmologists began to utilize anti-TNF-a for ocular inflammation ocular (25).
At present, anti TNF-a Drugs are usually reserved for immunosuppressant-resistant patients. For example, in the case of uveitis associated to juvenile idiopathic arthritis there is an official treatment protocol issued by the Spanish Society of Pediatric Rheumatology which has been agreed by the ophthalmologist and rheumatologists recommending treatment with anti TNF-a After the failure of corticosteroids and methotrexate. However, due to the advantages in efficacy and quality of life exhibited by TNF-a in comparison to typical immunosuppressants, it is increasingly common to introducing these drugs at an earlier stage in order to restrict structural damage as much as possible.
A range of scientific publications document the beneficial effect of anti TNF-a drugs in ocular and orbitary inflammations (26-31). In the case of Etanercept, some publications affirm it is an agent that produces uveitis (32,33). To this date, the use of Certolizumab for ocular inflammation has not been reported.
Finally, even though infliximab, adalimumab and etanercept exhibited potential usefulness in the field of uveitis and neuromodulation of apoptosic signals of retina ganglion cells, we must point out some potential neuro- ophthalmological adverse effects associated to various systemic administration in the form of anterior optic neuropathies and oculomotor nerve palsy (15,34,35). In addition, at the systemic level these drugs are known to increase the risk of infections, reactivation of latent tuberculosis, neoplasia, cardiovascular diseases and to the development of self immune diseases such as multiple sclerosis (which must be taken into account in the management of intermediate uveitis due to their association with this disease), as well as systemic erythematous lupus or sarcoidosis (36).
All the above leads us to continue analyzing the safety profile and potential usefulness of this group of drugs.
RESULTS OF ANTI-TNF-a DRUGS IN UVEÍTIS
Numerous articles have been published describing the benefits of anti TNF-a drugs for treating uveitis. They can be introduced in non-infectious uveitis resistant to corticoid and immunosuppressant treatment.
Beneficial effects have been described involving reductions in the number of relapses, diminished corticoid doses and improved disease control. The infusion of Infliximab in a dose of 3-10 mg/kg (in single or multiple dosis) for idiopathic uveitis or associated to juvenile idiopathic arthritis, ankylosing spondylitis, Beçhet’s disease, sarcoidosis and Crohn’s disease (37-40). This drug has also demonstrated its efficacy in the treatment of complications secondary to uveitis such as cystic macular edema (CME). In the case of Infliximab infusion in a dose of 5 mg/kg, positive results have been reported with improvements in visual acuity and macular thickness in patients without active uveitis and with long-standing CME (14 months) (41).
Other authors (42) reported beneficial effects both at the ocular and systemic level obtained by treating with Adalimumab in patients with posterior uveitis associated to sarcoidosis, resistance to treatment with corticoids and methotrexate. In what concerns uveitis associated to Beçhet’s disease, Adalimumab has also demonstrated good efficacy and has been successfully used as an alternative to Infliximab or as a substitute thereof in well controlled patients without diminishing the disease remission period (43).
Figures 1 and 2 portray a clinical case of progressive subretinal fibrosis in a female aged 32 where the progression of the disease was halted after treatment with Adalimumab.
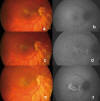
Fig. 1: Progression can be seen in
retinographies and autofluorescences (ačf) during
7 months despite treatment with prednisone, cyclosporine and azathioprine.
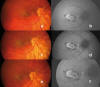
Fig. 2: Retinographies and
autofluorescences (ačf) show stabilization of the
lesion after the introduction of Adalimumab. Five months follow-up.
CONCLUSIONS
Anti-TNF-a are the most commonly used biological drugs in rheumatology. Due to a better understanding of the pathogenesis of ocular inflammation (both in cases associated to systemic diseases as well as in idiopathic cases), interest in these therapies has increased in ophthalmology as demonstrated by increased publications in this area. Articles published to date reports high efficacy of anti-TNF-a treatments for ocular inflammation. Possibly, the future development of a vehicle specifically designed for ocular administration make open new therapeutic possibilities for ocular inflammation or other ophthalmological pathologies.
Due to the advantages in terms of efficacy and quality of life said drugs exhibits vis-ŕ-vis typical immunosuppressants, it is increasingly common to introducing these drugs at an earlier stage with the aim of restricting structural damages as much as possible.
Anti-TNF-a Drugs exhibits a good safety profile although an adequate prior study of the patient must be carried out in cooperation with the rheumatologist or internist, who would be in charge of monitoring the follow-up of these patients.
REFERENCES
-
McDermott MF. TNF and TNFR biology in health and disease. Cell Mol Biol 2001; 47: 619-635.
-
Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther 2008; 117: 244-279.
-
Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med 1996; 334: 1717-1724.
-
Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D, Kollias G. Transgenic mice expressing human tumor necrosis factor: a predictive genetic model of arthritis. EMBO J 1991; 10: 4025-4031.
-
Feldmann M, Maini RN. Anti-TNF-a therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol 2001; 19: 163-196.
-
Sfikakis PP, Kollias G. TNF biology in experimental and clinical arthritis. Curr Opin Rheumatol 2003; 15: 380-386.
-
Fontaine V, Mohand-Said S, Hanoteau N, Fuchs C, Pfizenmaier K and Eisel U. Neurodegenerative and neuroprotective effects of tumor necrosis factor (TNF) in retinal ischemia: opposite roles of TNF receptor 1 and TNF receptor 2. J Neurosci 2002; 22: RC216.
-
Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ 2003; 10: 45-65.
-
Rath PC, Aggarwal BB. TNF-induced signaling in apoptosis. J Clin Immunol 1999; 19: 350-364.
-
Cunningham Jr ET, Stalder A, Sanna PP, Liu SS, Bloom FE, Howes Jr EL, Campbell IL, Margolis TP. Localization of tumor necrosis factor receptor messenger RNA in normal and herpes simplex virus-infected mouse eyes. Invest Ophthalmol Vis Sci 1997; 38: 9-15.
-
Sippy BD, Hofman FM, Wright AD, He S, Ryan SJ, Hinton DR. Soluble tumor necrosis factor receptors are present in human vitreous and shed by retinal pigment epithelial cells. Exp Eye Res 1996; 63: 311-317.
-
Smith JR, Hart PH, Williams KA. Basic pathogenic mechanisms operating in experimental models of acute anterior uveitis. Immunol Cell Biol 1998; 76: 497-512.
-
Ahmad T, Wallace GR, James T, Neville M, Bunce M, Mulcahy-Hawes K, Armuzzi A, Crawshaw J, Fortune F, Walton R, Stanford MR, Welsh KI, Marshall SE, Jewell DP. Mapping the HLA association in Behçet’s disease: a role for tumor necrosis factor polymorphisms? Arthritis Rheum 2003; 48: 807-813.
-
Grant MB, Afzal A, Spoerri P, Pan H, Shaw LC, Mames RN. The role of growth factors in the pathogenesis of diabetic retinopathy. Expert Opin Investig Drugs 2004; 13: 1275-1293.
-
Theodossiadis PG, Markomichelakis NN, Sfikakis PP. Tumor necrosis factor antagonists: preliminary evidence for an emerging approach in the treatment of ocular inflammation. Retina 2007; 27: 399-413.
-
Rojas J, Fernandez I, Pastor JC, Garcia-Gutierrez MT, Sanabria MR, Brion M, Coco RM, Ruiz-Moreno JM, Garcia-Arumi J, Elizalde J, Ruiz-Miguel M, Gallardo JM, Corrales RM, Carracedo A. A strong genetic association between the tumor necrosis factor locus and proliferative vitreoretinopathy: the retina 4 project. Ophthalmology 2010; 117: 2417-2423.
-
Calabrese LH. Molecular differences in anticytokine therapies. Clin Exp Rheumatol 2003; 21: 241-248.
-
Weisman MH, Moreland LW, Furst DE, Weinblatt ME, Keystone EC, Paulus HE, Teoh LS, Velagapudi RB, Noertersheuser PA, Granneman GR, Fischkoff SA, Chartash EK. Efficacy, pharmacokinetic, and safety assessment of adalimumab, a fully human anti-tumor necrosis factor-alpha monoclonal antibody, in adults with rheumatoid arthritis receiving concomitant methotrexate: a pilot study. Clin Ther 2003; 25: 1700-1721.
-
Boyce EG, Halilovic J, Stan-Ugbene O. Golimumab: review of the efficacy and tolerability of a recently approved tumor necrosis factor- inhibitor. Clin Ther 2010; 32: 1681-703.
-
Zhou H. Clinical pharmacokinetics of etanercept: a fully humanized soluble recombinant tumor necrosis factor receptor fusion protein. J Clin Pharmacol 2005; 45: 490-497
-
Kaushik VV, Moots RJ. CDP-870 (certolizumab) in rheumatoid arthritis. Expert Opin Biol Ther 2005; 5: 601-606.
-
Lacomba MS, Martin CM, Gallardo Calera JM, Gómez Vidal MA, Collantes Estévez E, Ramírez Chamond R, Omar M. Aqueous humor and serum tumor necrosis factor-a in clinical uveitis. Ophthalmic Res 2001; 33: 251-255.
-
Rahi AH, Al-Kaff A. Cytokine profile in uveitis: a point prevalence study. In: Whitcup SSM, Nussenblatt RB, Caspi RR, Gery I, eds. Advances in Ocular Immunology. International Congress Series. Amsterdam: Elsevier Science 1994: 369-372.
-
Palexas GN, Sussman G, Welsh NH. Ocular and systemic determination of IL-1 beta and tumour necrosis factor in apatient with ocular inflammation. Scand J Immunol Suppl 1992; 11: 173-175.
-
Rodrigues EB, Farah ME, Maia M, Penha FM, Regatieri C, Melo GB, Pinheiro MM, Zanetti CR. Therapeutic monoclonal antibodies in ophthalmology. Prog Retin Eye Res 2009; 28: 117-44.
-
Cordero-Coma M, Salom D, Díaz-Llopis M, López-Prats MJ, Calleja S. Golimumab for uveitis. Ophthalmology 2011; 118: 1892.
-
Ashok D, Ayliffe WH, Kiely PD. Necrotizing scleritis associated with rheumatoid arthritis: long-term remission with high-dose infliximab therapy. Rheumatology 2005; 44: 950-951.
-
Cazabon S, Over K, Butcher J. The successful use of infliximab in resistant relapsing polychondritis and associated scleritis. Eye 2005; 19: 222-224.
-
Diaz-Valle D, Miguelez Sanchez R, Fernández Espartero MC, Pascual Allen D. Treatment of refractory anterior diffuse scleritis with infliximab. Arch Soc Esp Oftalmol 2004; 79: 405-408.
-
El-Shabrawi Y, Hermann J. Anti-tumor necrosis factor-alpha therapy with infliximab as an alternative to corticosteroids in the treatment of human leukocyte antigen B27-associated acute anterior uveitis. Ophthalmology 2002; 109: 2342-2346.
-
Rubin PA, Foster CS. Etiology and management of idiopathic orbital inflammation. Am J Ophthalmol 2004; 138: 1041-1043.
-
Reddy AR, Backhouse OC. Does etanercept induce uveitis? Br J Ophthalmol 2003; 87: 925.
-
Fonollosa A, Artaraz J, Les I, Martinez-Berriotxoa A, Izquierdo JP, Saiz Lopez A, Gardeazaba J, Berasategui B, Martinez-Alday N. Sarcoid Intermediate Uveitis following Etanercept Treatment: A Case Report and Review of the Literature. Ocul Immunol Inflamm 2011; 21. [Epub ahead of print].
-
Chung J.H, Van Stavern GP, Frohman LP, Turbin RE. Adalimumab associated optic neuritis. J Neurol Sci 2006; 15: 133-136.
-
Suhler EB, Smith JR, Wertheim MS, Lauer AK, Kurz DE, Pickard TD, Rosenbaum JT. A prospective trial of infliximab therapy for refractory uveitis: preliminary safety and efficacy outcomes. Arch Ophthalmol 2005; 123: 903-912.
-
Ramos-Casals M, Perez Alvarez R, Diaz Lagares C, Cuadrado MJ, Khamashta MA, The BIOGEAS study group. Autoimmune diseases induced by biological agents: a double edge sword? Autoimmune Rev 2010; 9: 188-193.
-
Benitez-del-Castillo JM, Martinez-de-la-Casa JM, Pato-Cour E, Pato-Courm E, Méndez-Fernández R, López-Abad C, Matilla M, Garcia-Sanchez J. Long-term treatment of refractory posterior uveitis with anti-TNFalpha (infliximab). Eye 2005; 19: 841-845.
-
Deuter CM, Kötter I, Wallace GR, Murray PI, Stübiger N, Zierhut M. Behçet’s disease: ocular effects and treatment. Prog. Retin. Eye Res 2008; 27: 111-136.
-
Adán A, Hernandez V, Ortiz S, Molina JJ, Pelegrin L, Espinosa G, Sanmartí R. Effects of infliximab in the treatment of refractory posterior uveitis of Behçet’s disease after withdrawal of infusions. Int Ophthalmol 2010; 30: 577-81.
-
Bodaghi B, Bui Q, Wechsler B, Tran T, Cassoux N, Huong D, Thi L, Chosidow O, Herson S, Piette J, LeHoang P. Therapeutic use of infliximab in sight threatening uveitis: retrospective analysis of efficacy, safety, and limiting factors. Ann Rheum Dis 2005; 64: 962-964.
-
Markomichelakis NN, Theodossiadis PG, Pantelia E, Papaefthimiou S, Theodossiadis GP, Sfikakis PP. Infliximab for chronic cystoid macular edema associated with uveitis. Am J Ophthalmol 2004; 138: 648-650.
-
Erckens RJ, Mostard RL, Wijnen PA, Schouten JS, Drent M. Adalimumab successful in sarcoidosis patients with refractory chronic non-infectious uveitis. Graefes Arch Clin Exp Ophthalmol 2011; 27. [Epub ahead of print].
-
Mushtaq B, Saeed T, Situnayake RD, Murray PI. Adalimumab for sight-threatening uveitis in Behçet’s disease. Eye 2007; 21: 824-825.