UPDATE REVIEW
Ocular pharmacology in pediatrics
GIL RUIZ MR1, ORTEGA USOBIAGA J2, PÉREZ ANDÚJAR JÁ3, GIL RUIZ MI4, NEVADO CABALLERO A5
1 Ph.D in
Medicine and Surgery. Deputy Physician, Ophthalmology Service. General Hospital
Nuestra Señora del Prado. Talavera de la Reina (Toledo, Spain).
2 Ph.D in Medicine and Surgery. Specialist in ophthalmology. Baviera
Clinic. European Ophthalmological Institute. Bilbao (Vizcaya, Spain).
3 Graduate in Medicine and Surgery. Deputy Physician, Anesthesiology
and Reanimation Service. General Hospital Nuestra Señora del Prado. Talavera de
la Reina (Toledo, Spain).
4 University Diploma in Nursing. University Hospital San Agustín.
Linares (Jaén, Spain).
5 Graduate in Medicine and Surgery. Chief, Ophthalmology Service.
General Hospital Nuestra Señora del Prado. Talavera de la Reina (Toledo, Spain).
INTRODUCTION
The best clinical utilization of a drug should consider the efficacy as well as the safety of each molecule. The differences in these 2 areas between pediatric and adult patients are due to the changes in pharmakinetics and dynamics involved in the development of the human body. These changes peak in newborns who generally exhibit rates of absorption, protein bonding, metabolism and excretion of drugs lower than adults in contrast with higher distribution volumes (1).
Significant errors in medicine have been caused by erroneously considering children as small adults, including hearing loss due to the use of aminoglycosides or newborn gray syndrome due to chloramphenicol. It is not possible to extrapolate research carried out in adults into the pediatric area. The World Health Organization (WHO) states that clinical trials in children are essential to develop therapies and interventions specifically for this age group as well as to determine and optimize the best available medical treatments. Ethical and clinical aspects must be taken into account in the design, implementation and assessment of said clinical trials and their results. In this sense, since late 2007 the WHO is developing the «Medications Made for Kids» campaign in an effort to increase research in this field so that many existing medications can be developed for children and made available in the adequate form and dosage.
The FDA (Food and Drug Administration) is the US government agency in charge of regulating biological products, food supplements, cosmetics, medical devices, hematic products, food and drugs (these two for humans as well as animals).
Said agency regulates the marketing of pharmaceuticals, assessing consumer safety and efficacy of active principles. Assuming that children do not exhibit the same anatomical, physiological and biochemical characteristics, the FDA has divided the pediatric population in 5 age groups: intrauterine, newborn (up to month one of life), infant or in lactation (up to age 2), childhood (up to the beginning of puberty) and adolescent (from puberty to adulthood). The dosage of medications should be adjusted by age, weight and condition of the disease, among other relevant factors.
In pediatrics, the administration of medication exhibits not only bioavailability changes in different age groups; it can also disturb the normal growth of a child and affect the end result of biological development. Any mistake in the dosage adjustment could render a drug ineffective or even worse, unsafe and toxic.
Side effects may arise in children while utilizing topical drugs in the eyes provided that systemic absorption is relevant. The ophthalmic pathway should not be underestimated in this regard. Even so, we must differentiate the occasional use of eyedrops that can be relatively safe with continued use which, in certain cases, could be contraindicated due to the risk of toxicity.
In what concerns the systemic absorption of eyedrops, clinical cases have been reported that emphasize the importance of this administration pathway. Kidney failure in a low weight newborn was reported after the use of topical phenylephrine for exploring retinopathy of prematurity (2). The report concludes that topical midriatic drugs should be used in newborns with precaution, pressing the lachrymal pathway in order to avoid systemic absorption and monitoring the patient during the administration of the drops and while their effect subsists.
The smallest amount of eyedrops that can be instilled is one drop, which is adequate for an adult but excessive for a newborn that requires only 50% of said amount, or a 3-year-old child that needs 75% or a six-year-old who would have enough with 90% of said dose (3). This fact, together with a smaller volume of blood and the immaturity of the enzymatic system and the blood-brain barrier, involve higher plasmatic concentrations of the active principle and unforeseeable side effects.
In order to remain focused on the purpose of this paper, i.e., to establish useful basic guidelines for daily clinical practice in ophthalmology and pediatrics, this review aims at providing a general overview of the various pharmacological groups most widely used in children. To obtain detailed information about active principles, it is recommended to refer to specialized sources.
1. MIDRIATICS
Together with antibiotics and antihistaminics, this pharmacological family is widely used in children. Topical midriatics can produce midriasis and cyclopegia or only midriasis. Within the first group the main drugs are: atropin, the most powerful and long-lasting drug (7-14 days) followed by scopolamine (3-5 days), homatropine (24-36 hours), cyclopentholate (12-24 hours) and the short-lived tropicamide (4-6 hours) (4). In the group of the adrenergic sympathetic system agonists we have phenylephrine (6-8 hours). Of all these, the most adequate medication for funduscopy and refractive study in pediatrics is cyclopentholate due to its short-lived effect and ability to stop accommodation.
The systemic action of atropine is dosage dependent and its side effects can be extremely severe, ranging from bradychardia and sweating to ataxia and coma (5).
Atropin is applied in the treatment of amblyopia to penalize the predominant eye, and is effective and well tolerated in cases where the occlusions are not effective (6).
In what concerns cyclopentholate, nervous system alterations have been described including hallucinations, disorientation, memory loss and confusion. In addition to being dose- dependent, said side effects also seem to reveal an individual predisposition caused by receptor hypersensitivity (5). In addition, involvement of the gastrointestinal system has been described, even necrotizing enterocolitis in premature newborns (7).
At any rate and as commented above, the sporadic use of an active principle for ocular fundus exploration, which can be relatively safe, should be differentiated from prolonged use.
Homatropin and scopolamin are hardly used in practice, the former because it does not produce adequate cycloplegia in children and the latter because it exhibits the same secondary effects as atropin but with greater frequency (6).
As stated in the introduction, kidney failure in a low weight newborn was reported after the use of topical phenylephrine for exploring retinopathy of prematurity (2). For this reason, topical midriatic drugs should be applied in newborns with special care, pressing the lachrymal pathway to prevent systemic absorption and monitoring the patient during the application of the drops and while its effect subsists.
During lactation all midriatic drugs should be used with precaution, avoiding entirely phenylephrine and scopolamine (8).
2. HYPOTENSORS
Glaucomatous optic neuropathy is an important cause of blindness in children and adults. Congenital glaucoma affects one out of every 10,000 live newborns (3) and even though the treatment of congenital glaucoma is always surgical, the use of drugs can be useful prior to goniotomy or while awaiting surgery.
The only families of hypotensors approved by the FDA for pediatric glaucoma patients are beta- blockers, carbonic anhydrase inhibitors and miotics (9). The main drawback in these patients is the high frequency of secondary effects due to high concentrations of the active principle in the blood which can be reached due to the difficulty in calculating the topical dosage. By including the lachrymal points, it is possible to diminish systemic absorption. On the other hand, systemic treatments can be applied with greater dosage control on the basis of patient weight, making these extremely recommendable in smaller children.
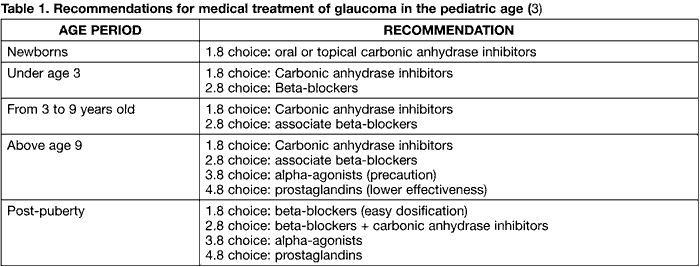
Table 1 illustrates a scheme for hypotensor medical treatment guidelines in pediatrics. Carbonic anhydrase inhibitors are the treatment of choice in newborns, with the oral pathway being preferred over topical pathway as the dosage is more easily calculated. If beta-blockers are necessary, cardioselective molecules such as betaxolole are preferred for asthmatic patients. Prostaglandins turned out to be less efficient in children than in adults, although in juvenile glaucoma and with older children these drugs are able to significantly reduce intraocular pressure (10).
Alpha-adrenergic drugs should not be utilized as they could bring about severe central nervous system depression. Brimonidine can cause miosis, hyperthermia, dizziness, agitation, ataxia, drowsiness, coma, apnea, hypo- and hypertension, bradycardia, respiratory depression and death (11).
Poisoning is frequent in children, in 84.3% due to indigestion, 5.7% through the ocular pathway, 3.4% through the skin and in the rest of cases the pathway is unknown (11). Naloxone, an opiate antagonist, is used as an antidote in severe cases.
The American Pediatric Academy has approved the administration of beta blockers and carbonic anhydrase inhibitors during lactation, although with precaution. Alpha-adrenergic agonists should be avoided as they are contraindicated for the pediatric age (12).
3. ANTIBIOTICS
The list of anti-infectious drugs available at present for ophthalmic use is very long. Bacterial conjunctivitis and corneal ulcers, as well as keratitis due to traumatism and foreign bodies are quite frequent in ophthalmological and pediatric practices. In addition, Ophthalmia Neonatorum, i.e., muco-purulent acute conjunctivitis in the first month of life and acquired in the birth canal, appears in 1-12% of newborns. This ophthalmia became so relevant that it was necessary to establish prophylactic protocols for all newborns, applying 1% nitrate solution, 1% tetracycline solution, 1% erythromycin solution, 2.5% iodine povidone or fusidic acid (13).
Glycopeptides (vancomycin and teicoplanin) are not frequently utilized in ophthalmology and are reserved for severe ocular infections. Specifically, vancomycin is utilized as reinforced eyedrops for corneal conditions and through the intravitreous pathway in endophthalmitis (14).
Parenterally applied aminoglycosides are othotoxic and nephrotoxic. With the exception of tobramycin, neomycin and gentamicin, which exhibit a certain degree of safety, the rest should be avoided for the aforementioned reasons.
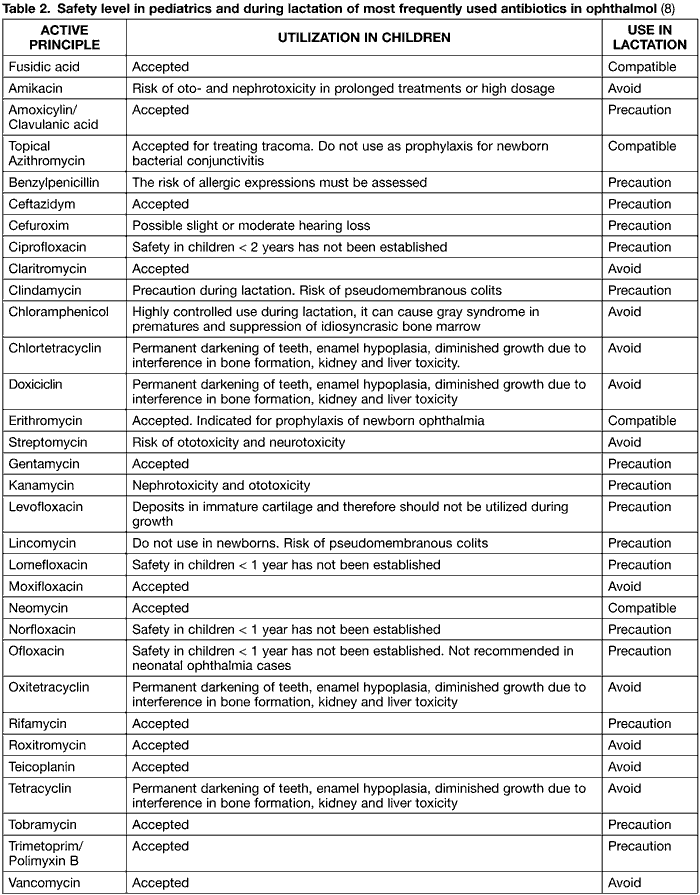
The administration of tetracyclines is contraindicated in lactation and in patients under 8 years of age. These active principles are excreted through maternal milk and in children can account for permanent darkening of teeth, inanimate hypoplasia, delays in osteogenesis and kidney and liver toxicity (14) (table 2).
The use of chloramphenicol during lactation should be strictly controlled as it can cause the "gray baby syndrome" in premature babies and idiosyncratic bone marrow suppression (15).
Macrolides constitute a pharmacological group which is frequently used in pediatrics. Erythromycin can be utilized for preventing Ophthalmia Neonatorum. Topical azithromycin is efficient and safe for oral administration in tracoma treatment for children (16), even though it should not be included in a protocol for prophylaxis of neonatal conjunctivitis.
Lincosamides (lincomycin and clindamycin) should not be utilized in newborns due to the risk of pseudomembranose colits (8). Fusidic acid is probably the new promise for treating Ophthalmia Neonatorum, even though there are not yet sufficient data (16).
Of the 4 quinolone generations, the most widely used in ophthalmology are lomefloxacin, norfloxacin, ofloxacin, ciprofloxacin, levofloxacin, moxifloxacin and gatifloxacin. In general, these quinolones have not been studied in infants under age 1 and should not be utilized in pediatrics because they deposit in immature cartilage and affect growth (14). The only quinolone that has exhibited clinical safety in 0.5% ophthalmic solution is moxifloxacin (17) since day 3 after birth.
It is allowed to prescribe trimetoprim associated to polymixin B for treating ocular surface infections during the pediatric period (8).
In what concerns lactation, with the exception of fusidic acid, erythromycin and neomycin which have demonstrated compatibility, the rest of antibiotics should be used with precaution or avoided altogether (table 2).
4. ANTIALLERGICS AND VASOCONSTRICTORS
Ocular allergy affects 25% of the general population in developed countries and includes a group of pathologies which can affect different areas of external ocular surfaces (18). The first contact with the allergene usually occurs in early stages of childhood and for this reason it is crucial for pediatricians to have a sound knowledge of therapeutic strategies. Frequently, a multidisciplinary approach is required for controlling chronic and severe forms of allergy.
The antiallergic drugs comprise various families: histamine receptor antagonists, mastocyte degranulation inhibitors and multi-action drugs (table 3).

The first group inhibits histamine receptors in a competitive but not selective manner, inhibiting also cholinergic and serotonine-synergic receptors, among others. This gives rise to adverse effects (18). Levocabastin can be utilized during lactation whereas chlorphenamin and emedastin require precaution in their use (8).
Within the mastocyte degranulation inhibitors group, spaglumic acid should be avoided in lactating mothers, prescribing disodium chromoglycate and ldoxamide and nedochromyl, these two with precaution. Chromoglycate and nedochromyl exhibit minimum ocular and systemic absorption. This active principle should be used in moderation in patients aged between 3 and 6 due to limited clinical experience (8). Despite this recommendation, a comparative, placebo and double blind study has been published comprising 2905 patients between 3 and 76 years of age. The researchers concluded that nedochromyl is more effective than placebo and exhibits good tolerance without relevant side effects (19).
Internal, multi-action drugs exhibit antihistaminic activity which stabilizes the mastocyte membrane as well as anti-inflammatory activity. Olopatadin should be avoided in lactation, prescribing instead ketotifen and, exercising care, azelastin and epinastin (8). Azelastin is particularly suited for treating seasonal allergic conjunctivitis after age 4 and in perennial allergic conjunctivitis after age 12.
Topical vasoconstrictor drugs (simpathomimetic) are prescribed as a symptom relief for conjunctival hyperemia. Phenylephrine, nafazoline and oxymetazoline can be utilized with precaution by women during lactation. In contrast, tetrizoline should be avoided. The latter active principle is used indiscriminately by the population even though it is true that it is not recommended for infants under 2 years of age. Nafazoline and oxymetazoline should not be utilized in children under 5-6 years of age (8). Phenylephrine as a vasoconstrictor agent is presented in concentrations below those required for achieving midriasis.
5. ANTI-INFLAMATORY DRUGS
Inflammation is a nonspecific, defensive and repairing response of vascularized connective tissue. When this process becomes permanent, it could cause damages. At this point is when anti- inflammatory drugs (corticoids and non-steroids) intervene by inhibiting said response (20).
The pediatric age is highly sensitive to corticoids, with enhanced susceptibility for the development of posterior subcapsular cataracts or significantly increased intraocular pressure. In practice, the topical administration of these active principles does not associate systemic adverse effects, providing a false feeling of safety and facilitating indiscriminate use. This increases the risk of opportunistic infections and the above-mentioned ocular toxicity. For these reasons, ongoing therapy in children should be avoided (21). Through the systemic pathway and in chronic treatments, adrenal inhibition could ensue.
Fluormetholone requires special care if utilized in infants under 2 years of age, while rimexolone is not advised. In general, corticoids should be avoided during lactation although dexametasone is permitted (8) (table 4).
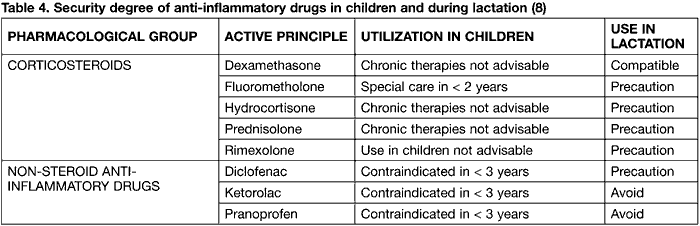
As regards non-steroid anti-inflammatory drugs, these are not allowed for children under 3 years of age. Diclofenac can be prescribed during lactation with precaution, whereas ketorolac and pranoprofen are prohibited (8).
6. OTHER INFLAMMATION-MODULATING AGENTS
Cyclosporin A is a selective immunosuppressant drug which inhibits immunocompetent cells without generating direct cytotoxic effects. Of all its possible indications, it is frequently utilized in ophthalmology practices and in children to treat vernal and atopic keratoconjunctivitis and subepithelial corneal infiltrates after infectious conjunctivitis.
It is not known if its topical administration involves active principle excretion in maternal milk; for this reason it is not advisable to prescribe it during lactation (21).
The use of topical cyclosporin A in children is allowed because its efficacy and safety has been confirmed by several studies, even at concentrations of 2% (22,23) and in long treatments. Even so, the possibility of opportunistic infections should be closely analyzed (24).
REFERENCES
-
Flórez J. Farmacología humana. 3.ª edición; Barcelona: Masson; 2000.
-
Shinomiya K, Kajima M, Tajika H, Shiota H, Nakagawa R, Saijyou T. Renal failure caused by eyedrops containing phenylephrine in a case of retinopathy of prematurity. J Med Invest 2003; 50: 203-206.
-
Fernández-Vidal AM, Méndez CD, Martínez de la Casa JM, García-Feijoó J. Tratamiento médico del glaucoma congénito. En: Cortés C, Arias A, Encinas JL, García-Feijoó J. LXXXIII Ponencia Oficial de la Sociedad Española de Oftalmología. Farmacología Ocular. Madrid: Industria Gráfica MAE SL; 2007: 285-286.
-
Cortés C, Cuesta T, Gil MR. Fármacos midriáticos: pautas de dilatación en cirugía de cristalino. En: Lorente R, Mendicute J. LXXXIV Ponencia Oficial de la Sociedad Española de Oftalmología. Cirugía del Cristalino. Madrid: Industria gráfica MAC LINE SL; 2008; I: 421-427.
-
Encinas JL, Cajigal C. Midriáticos. Colorantes. En: García-Sánchez J, García-Feijoó J. Oftalmomecum 2009. Barcelona: P. Permanyer; 2009: 11-18.
-
Cortés C, Cortés I, Barrera C. Midriáticos y ciclopléjicos. En: Cortés C, Arias A, Encinas JL, García-Feijoó J. LXXXIII Ponencia Oficial de la Sociedad Española de Oftalmología. Farmacología Ocular. Madrid: Industria Gráfica MAE SL; 2007: 69-76.
-
Bauer R, Trepannier MC, Stem L. Systemic cyclopentolate toxicity in the new born infant. Journal of pediatric Pharmacology and Therapeutics 1973; 82: 501-505.
-
Vademecum Internacional. 52ª edición; Madrid: CMP Medicom; 2011.
-
Maris PJ, Mandal AK, Netland PA. Medical therapy of pediatric glaucoma and glaucoma in pregnancy. Ophthalmol Clin North Am 2005; 18 (3): 461-468.
-
Enyedi LB, Freedman SF. Latanoprost for the treatment of pediatric glaucoma. Surv Ophthalmol 2002; 47 Suppl 1: S129-132.
-
Becker ML, Huntington N, Woolf AD. Brimonidine tartrate poisoning in children: frequency, trends, and use of naloxone as an antidote. Pediatrics 2009; 2: 305-311.
-
Urcelay JL. Tratamiento médico antiglaucomatoso en mujeres gestantes y lactantes. En: Cortés C, Arias A, Encinas JL, García J. LXXXIII Ponencia Oficial de la Sociedad Española de Oftalmología. Farmacología Ocular. Madrid: Industria Gráfica MAE SL; 2007: 297-299.
-
Zuppa AA, D´Andrea V, Catenazzi P, Scorrano A, Romagnoli C. Ophthalmia neonatorum: what kind of prophylaxis? J Matern Fetal Neonatal Med 2011; 24 (6): 769-773.
-
De las Heras B, Arias A, Bañuelos J, García C. Antibióticos. En: Cortés C, Arias A, Encinas JL, García-Feijoó J. LXXXIII Ponencia Oficial de la Sociedad Española de Oftalmología. Farmacología Ocular. Madrid: Industria Gráfica MAE SL; 2007: 77-108.
-
Arias A, García MC, Matilla M. Antiinfecciosos. Antimicrobianos, antifúngicos y antivirales. En: García-Sánchez J, García-Feijoó J. Oftalmomecum 2009. Barcelona: P. Permanyer; 2009: 19-50.
-
Cochereau I, Goldschmidt P, Goepogui A, Afghani T, Delval L, Pouliquen P, Bourcier T, Robert PY. Efficacy and safety of short duration azithromycin eye drops versus azithromycin single oral dose for the treatment of tracoma in children: a randomised, controlled, double-masked clinical trial. Br J Ophthalmol 2007; 91 (5): 667-672.
-
Silver LH, Woodside AM, Montgomery DB. Clinical safety of moxifloxacin ophthalmic solution 0,5% (Vigamox®) in pediatric and nonpediatric patients with bacterial conjunctivitis. Surv Ophthalmol 50; 2005: S55-S63.
-
Gil MR, Cortés C. Antialérgicos y vasoconstrictores. En: García-Sánchez J, García-Feijoó J. Oftalmomecum 2009. Barcelona: P. Permanyer; 2009: 65-70.
-
Kjellman NI, Stevens MT. Clinical experience with Tilavist: an overview of efficacy and safety. Allergy 1995; 50 (21 Suppl): 14-22.
-
Gil MR, Cortés C. Farmacología de la inflamación en Oftalmología. En: Cortés C, Arias A, Encinas JL, García-Feijoó J. LXXXIII Ponencia Oficial de la Sociedad Española de Oftalmología. Farmacología Ocular. Madrid: Industria Gráfica MAE SL; 2007: 163-178.
-
Arriola P, Díaz D, Benítez del Castillo JM. Antiinflamatorios. Corticosteroides, antiinflamatorios no esteroideos, otros agentes moduladores de la inflamación. En: García-Sánchez J, García-Feijoó J. Oftalmomecum 2009. Barcelona: P. Permanyer; 2009: 51-63.
-
Tatlipinar S, Akpek EK. Topical cyclosporine in the treatment of ocular surface disorders. Br J Ophthalmol 2005; 89: 1363-1367.
-
Foulks GN. Topical cyclosporyne for treatment of ocular surface disease. Int Ophthalmol Clin 2006; 46: 105-122.
-
Takamura E, Uchio E, Ebihara N, Okamoto S, Kumagai N, Shoji J, Nakagawa Y, Namba K, Fukushima A, Fujishima H, Miyazaki D, Ohasi Y. A prospective, observational, all-prescribed-patients study of cyclosporine 0,1% ophthalmic solution in the treatment of vernal keratoconjunctivitis. Nihon Ganka Gakkai Zasshi 2011; 115 (6): 508-515.