UPDATED REVISION
Ocular pharmacology during gestation and breastfeeding (I)
GIL RUIZ MR1, ORTEGA USOBIAGA J2, GIL RUIZ MT3, CORTÉS VALDÉS C4
1
PhD in Medicine and Surgery. Ophthalmology Service Deputy. Nuestra Señora del
Prado Hospital. Talavera de la Reina (Toledo, Spain).
2
PhD in Medicine and Surgery. Ophthalmology Specialist. Baviera Clinic –European
Ophthalmological Institute. Bilbao (Bizkaia, Spain).
3
PhD in Medicine and Surgery. Clinical Analysis Service Chief. Nuestra Señora del
Prado Hospital. Talavera de la Reina (Toledo, Spain).
4
PhD in Medicine and Surgery. Ophthalmology Dept. Chief. Hospital Gregorio
Marañón General University Hospital (Madrid, Spain).
INTRODUCTION
Even though the use of topical ocular drugs during pregnancy is a relevant issue, the information to be found in scientific literature is scarce. The fact that very few pregnant women relate the effect topical medication may have on the fetus, in addition to the large sample size which would be needed to conclude that a secondary effect is teratogenous and that researchers cannot include pregnant women in their clinical studies for ethical reasons, are but a few reasons why the term «this drug is safe for pregnant women» is controversial (1). Evidence could be found in animals or isolated clinical cases of undesirable effects, but there are no controlled randomized studies. The importance of this issue is obvious considering that, according to a study of the Canadian population, 1 out of every 5 pregnant women are exposed to drugs classified in category C, D or X (United States Food and Drug Administration classification) (2).
Following the Latin precept primum non nocere, we should be knowledgeable on the drugs we administer to pregnant women because they could cause severe and/or irreversible consequences for the fetus. In plain language, ‹the treatment should not be worse than the disease›.
Secondary effects could arise in the fetus when utilizing topical drugs in the eye when systemic absorption is relevant. For this reason, we should not underestimate the ophthalmic pathway although we must differentiate between the occasional use of eye drops, which can be relatively safe, to continued use which, in given cases, could be contraindicated due to teratogenic risks. Cases of severe embryonary malformations have been described after the use of 0.1% or 3% ocular topical EDTA taken six times a day, although said effects are unlikely to arise with a single drop (3).
In what concerns systemic absorption of instilled eye drops, clinical cases have been described which confirm the importance of this pathway. One article described the case of kidney failure in a low weight newborn after the use of topical phenylephrine for the exploration of premature retinopathy (4), concluding that topical midriatic drugs must be used in newborns with caution, pressing the lachrymal pathway to avoid systemic absorption and monitoring the patient during the instillation of the drops and while its effects subsist.
In addition, it must be remembered that before placing a new drug in the market, pharmaceutical companies hardly ever test the active principle in pregnant women, making it difficult to determine its effects on fetuses. Besides, to what extent can we extrapolate to humans the results obtained in animals? The case of misoprostol is an example of the possibility, however remote, of a drug causing damages to humans but not to animals (5).
Establishing clinical practice habits based on scientific evidence is essential. However, studies on the safety of ocular drugs in gestating or lactating patients are difficult to carry out due to the impossibility of making clinical trials with pregnant women for ethical reasons and due to the high number of variables intervening in embryo-fetal toxicity and teratogeny. These factors make it very difficult to establish therapeutic protocols during pregnancy which are exclusively based on scientific evidence because the lack of the latter does not necessarily imply a lack of efficiency. Evidence-based practice consists in «integrating individual clinical experience with the best external evidence» (6). We will never have randomized clinical trials for addressing every clinical issue, risk factor or preventive measure, but we can bring together what is reasonable and the best of scientific evidence in order to derive a set of efficient measures for practical application (7).
However, a revision of all the articles published between 1996 and 2003 on the potential risks of maternal ocular medication for the fetus (1) concluded that the risk of topically administered drugs on pregnant women is low, above all if utilized in a sporadic, non-continuous manner.
OCULAR CHANGES IN PREGNANCY
Gestation can give rise to various physiological changes in organs and systems, including the eyes. It could diminish corneal sensitivity (8) and thickness (9), increase its curvature, generate intolerance to contact lenses, diminish intraocular pressure levels (particularly in the latter months) and induce refractive changes (10). All of these changes gradually disappear in the postpartum period. In addition, at the pathological level pregnancy can also account for the appearance or progression of some diseases. It can alter the course of pre-existing diseases as well as develop specific pregnancy-related pathologies such as cortical blindness, visual anomalies derived from pre-eclampsia/eclampsia or precipitate the emergence of pathologies not related to pregnancy, such as central serous chorioretinopathy (11). Thee pathologies must be treated, and to do so it is necessary to know about the therapeutic options we are allowed to apply on these patients, taking great care to avoid embryo-fetal toxicity, teratogeny or even the possibility or activating a spontaneous miscarriage.
In what concerns the impact of pregnancy on pre-existing diseases, uveitic crises generally arise more frequently during the first quarter of the pregnancy or postpartum. Another concept to be considered as that the course of uveitis during pregnancy cannot be foreseen on the basis of the pre-gestation evolution of the inflammatory process (11).
Preeclampsia, a high pressure, pregnancy-specific condition and its association to the convulsion crises known as eclampsia, can associate visual symptoms. Diminished vision, phosphenes and visual field defects frequently include focal or diffuse construction of the retinal arterioles. The visual prognosis of these cases is good, with VA recovery after resolving the condition at the systemic level (12). All these details should be know to the obstetrician not only for early referral to the ophthalmologist but also as signs alerting of possible hypertension conditions of the pregnant woman. On the other hand, when the ophthalmologist is faced with these symptoms, particularly if the pregnant patient is at gestation week 20 or over, he should immediately report them to the obstetrician due to the severity of the process before beginning the exploration.
Cortical blindness is an unusual expression of preeclampsia/eclampsia derived from vascular compromise in the occipital cortex. Frequently, this loss of vision is preceded or accompanied by headaches and although in some cases these have lasted 192 hours, they generally disappear without leaving sequels (13).
Pregnancy is an important factor that gives rise to central serous chorioretinopathy. Even though macular serous detachment resolves in the first two months postpartum, the risk of relapse after gestation is higher after said period (11).
From the above comments the conclusion can be drawn that only with a profound knowledge of the impact of gestation on ocular physiology and pre-existing diseases as well as of the safety of pharmacological principles it is possible to provide adequate advice and treatment for pregnant women.
SAFETY CATEGORIES OF DRUGS ACCORDING TO THE FDA
The FDA is the United States government agency in charge of regulating biological products, food supplements, cosmetics, medical devices, hematic products, food and drugs (these two for humans and animals). It regulates the marketing of drugs and confirms their safety for consumers as well as the efficacy of the active principles. The FDA requires each new medication to overcome the four stages of clinical trials for safety and efficacy. The third stage, which is crucial for the future sale of a chemical substance, involves tests in 1,000-3,000 patients, with the research being stopped if any relevant adverse effects arise.
The FDA has designed safety categories based on the risk exhibited by a drug (table 1). There are 5 levels (14):
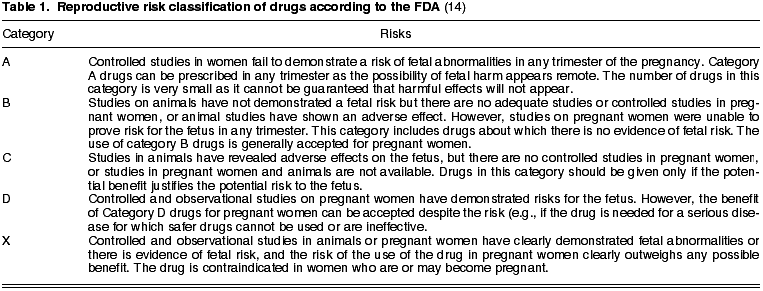
a) Category A: controlled studies have not exhibited risks
b) Category B: no tests evidence risk for humans.
c) Category C: the existence of risk cannot be discarded.
d) Category D: Evidence of risk for human fetus has been exhibited. The use of the drug by pregnant women is justified when vital risk exists or when safer drugs cannot be prescribed or have been proven to be inefficient.
e) Category X: studies in animals or humans have exhibited fetal alterations or evidence of fetal risk on the basis of human experience, or both, with risks clearly exceeding benefits. Contraindicated for pregnant women.
As a general rule, medication should not be utilized during pregnancy due to the possibility that it may cross the placenta barrier and damage the fetus. The first trimester is when the fetus is most vulnerable as most organs are formed during that period. In clinical practice, the use of category A drugs are accepted –very few at present– or category B in the absence of safer alternatives, in all cases under medical supervision (15).
Some drugs can be excreted in the mother’s milk, involving potential danger for the lactating infant.
The active principles that are compatible with breastfeeding are those which do not enter the mammary glands, which do not produce adverse effects on the lactating baby, or those which, while being excreted through said glands are not expected to cause any negative effect due to their characteristics (15).
The levels of safety in gestation and lactation for the drugs most commonly utilized in ophthalmology are described below. The letters A, B, C, D and X in brackets indicate the safety category of the active principle as per the FDA. In order to keep in mind the main objective of this revision (describe basic guidelines for daily clinical ophthalmological and primary health care practice), a general overview will be given for a range of pharmacological groups. In order to obtain more detailed information of the active principles it is suggested to consult specialized sources.
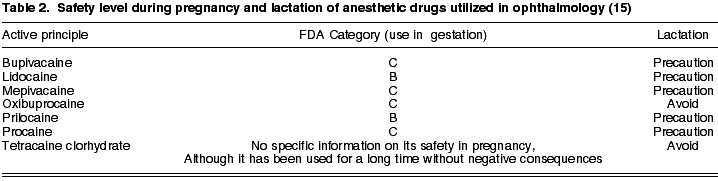
1. Anesthetics
Depending on the field of action and administration pathway, these drugs are classified as regional or topical anesthetics. The most widely used regional anesthetics are: procaine (C), lidocaine (B), mepivacaine (C), prilocaine (B) and bupivacaine (C). The most relevant topical anesthetics include oxibuprocaine (C) and tetracaine clorhydrate (15). No specific information about the safety of tetracaine in pregnancy is available, although it has been utilized for many years without negative consequences (15).
In general, anesthetics must be used with caution during lactation, endeavoring to avoid the use of tetracaine due to the lack of sufficient information (Table 2).
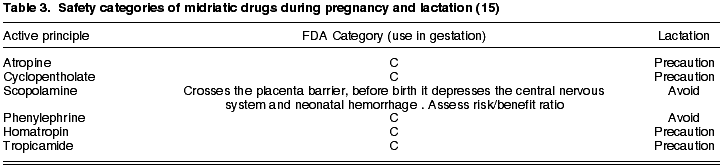
2. Midriatics
Topical midriatics can produce midriasis and cyclopegy or only generate midriasis. Of relevance in the first group we have atropine as the most potent and long-lasting drug (7-14 days), followed by scopolamine (up to 3-5 days), homatropin (24-36 hours), cyclopentholate (12-24 hours) and tropicamide, having the shortest duration (4-6 hours) (16). Of all these anesthetics, the most adequate for funduscopy in pregnancy is tropicamide due to its short-lived effect (17). The group of adrenergic sympathetic system agonists comprises phenylephrine (6-8 hours).
As discussed above, the one-off use of an active principle for ocular fundus exploration must be differentiated from extended use. Even so, the FDA classifies all these drugs in category C, which means that they can be administered only when the likely desired benefit justifies the potential risk for the fetus (14), with the exception of scopolamine which should be avoided as it traverses the placenta barrier and could depress the central nervous system and facilitate neonatal hemorrhage.
Cases of teratogeny have been described in association with parasympathetic-mimetic drugs (18) which, even when not administered via the ophthalmic pathway, had undesirable effects due to systemic absorption and passage to the fetus through the placenta. For this reason, authors advise dilating pregnant women with topical phenylephrine.
During lactation, all midriatic drugs must be utilized with caution, avoiding phenylephrine and scopolamine (Table 3).
3. Dyes
Dyes are utilized in ophthalmology to diagnose chorioretinal diseases or those of the conjunctiva, cornea, lachrymal pathway or chorioretinal. There is a broad range of pharmacological dyes such as sodium fluorescein, Bengal rose, methylene blue, alcyan blue, triphan blue, lisamine green, indocyanine green, proflavine, mercurochrome, argyrole and gentian purple (19). Fluorescein is the most widely used.
Even though topical fluorescein is safe during pregnancy and non-irritating, its teratogenic effects with intravenous administration are not known. Despite the innocuity of fluorescein during pregnancy referred in many articles (20), the risk/benefit ratio must be assessed for gestating or lactating patients.
As for the rest of staining active principles, there is a lack of experience in gestation to reach a reliable conclusion.
4. Antibiotics
At present there is a large amount of anti-infection drugs available for ophthalmic use. Drugs utilized during gestation should not exceed the FDA categories A and B. Accordingly, of the frequently used antibiotics in ophthalmology, the only one which exhibited the A security level is fusidic acid. Level B includes all the penicillins (such as amoxicillin and benzylpenicillin) and cephalosporines (ceftazydime and cefuroxyme, among others), tobramicine as the only aminoglycoside, erithromycin, azitromycin and roxithromycin in the macrolides group, lincosamines (licomycin and clindamycin) and rifampycine as the antimicobacterian drug (Table 4).
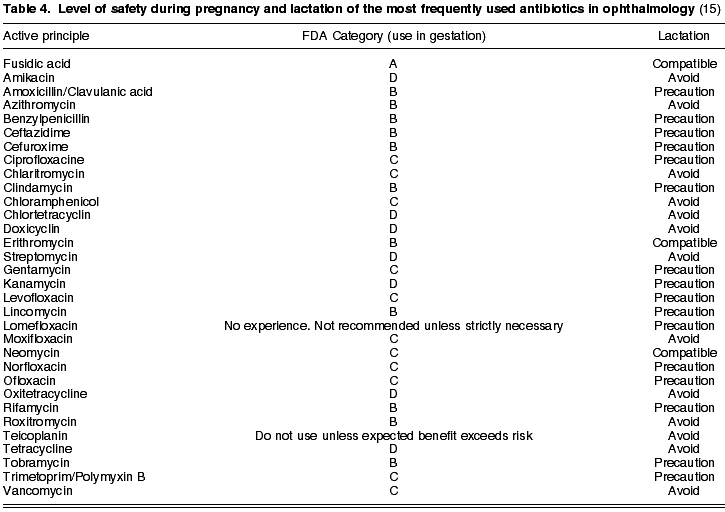
Glycopeptides (vancomycin and teicoplanine) are reserved for severe ocular infections. Specifically, vancomycin (C) is utilized as a reinforced eye drop for corneal conditions and intravitreous for endophthalmitis. Teicoplanine should not be prescribed during pregnancy unless the possible benefits exceed the risks.
Aminoglycosides easily penetrate the placental barrier (21) and, if administered parenterally, are ototoxic and nephrotoxic. With the exception of tobramycin, which has a B security level, aminoglycosides should be avoided as neomycin and gentamycin are C category, whereas streptomycin, amikacine and kanamycin are in the D category.
The administration of tetracyclines (D) is contraindicated during pregnancy, lactation and in patients under age 8. This pharmacological group exhibits the capacity of penetrating the placenta, accumulating in the teeth and bones of the fetus and being excreted in the mother’s milk (Table 4). In children it could be responsible for permanent darkening of the teeth and delays in osteogenesis (22).
The use of chloramphenicol (C) has produced reported cases of bone marrow depression and idiosyncratic aplasic anemia in non-gestating patients (21). For this reason it is not the first choice for pregnant patients.
Macrolides lack embryotoxic or teratogenous potential and exhibit an acceptable level of safety during gestation (21). Erithromycin, azitromycin and roxithromycin are in FDA Category A while clarithromycin is in category C (Table 4).
In turn, lincosamines (lincomycin and clindamycin) as all penicillins and cephalosporines, are in category B and therefore can be utilized with relative confidence during pregnancy. Fusidic acid is
The safest antibiotic is category A, but its reduced action spectrum limits its application. The four generations of quinolones are in level C. In ophthalmology, the most utilized members of this family are: lomefloxacin, norfloxacin, ofloxacin, ciprofloxacin, levofloxacin, moxifloxacin and gatifloxacin. Administered systemically, these antibiotics can reach therapeutic concentrations in the vitreous humor. Their use is not allowed in gestation, lactation and children because they deposit on immature cartilage (22).
Trimetoprim (category C) is associated to polymyxin B for treating ocular surface infections. Cotrimoxazol can produce pseudomembranous colitis and megaloblastic anemia due to folic acid deficit, and for this reason it is not recommended for pregnant women (22).
In what concerns lactation, with the exception of fusidic acid, erithromycin and neomycin which have been demonstrated to be compatible, the rest of antibiotics should be utilized with caution or avoided altogether (Table 4).
5. Antifungals
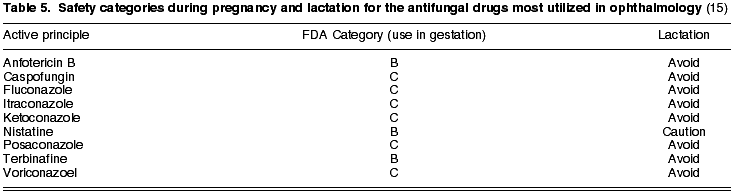
Antifungal antibiotics, utilized in keratitis caused by fungi and in fungal endophthalmitis, comprise five large groups: polyenes, azoles, alylamines, echinocandines and a miscellaneous group. The most frequently used in ophthalmology are: nistatin (B) and amphotericin B (B) in the polyene group: ketoconazol (C), itraconazol (C), fluconazol (C), voriconazol (C) and posaconazol (C) in the azole antifungals; terbinafine (B) as allylamine and caspofungine (C) representing the echinocandines (see Table 5). It is recommended to avoid all of these antibiotics during lactation, with the exception of nistatine, where caution is advised.
6. Antivirals
Virus are intra-cellular micro-organisms. This requires the drug to penetrate the invaded cell wall and search for the viral particle with higher or lower specificity. The toxicity of the active principles is a consequence of their capacity to differentiate the host from the virus (21).
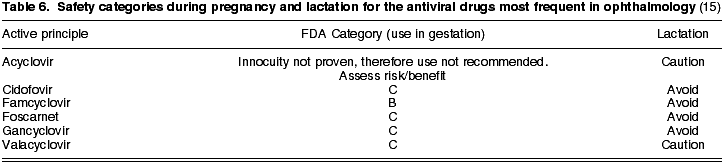
With the exception of famcyclovir, which is in Category B, the remaining antivirals -acyclovir, gancyclovir, valacyclovir, foscarnet, cidofovir- are in Category C. In addition, gancyclovir, famcyclovir, foscarnet and cidofovir must be avoided during lactation, while the remainder can be utilized after assessing the risk/benefit ratio, exercising caution in all cases (Table 6).
REFERENCES
-
Chung CY, Kwok AKH, Chung KL. Use of ophthalmic medications during pregnancy. Hong Kong Med J 2004; 10: 191-195.
-
Yang T, Walker MC, Krewski D, Yang Q, Nimrod C, Garner P, Fraser W, Olatunbosun O, Wen SW. Maternal characteristics associated with pregnancy exposure to FDA category C, D and X drugs in a Canadian population. Pharmacoepidemiology and Drug Safety 2008; 17: 270-277.
-
Gasset AR, Akaboshi T. Embryopathic effect of ophthalmic EDTA. Invest Ophthalmol Vis Sci 1977; 16: 652-654.
-
Shinomiya K, Kajima M, Tajika H, Shiota H, Nakagawa R, Saijyou T. Renal failure caused by eyedrops containing phenylephrine in a case of retinopathy of prematurity. J Med Invest 2003; 50: 203-206.
-
Koren G, Pastuszak A, Ito S. Drug Therapy. TNEJM 1998; 338: 1128-1137.
-
Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. BMJ 1996; 312: 71-72.
-
Schein OD. Prevention of endophthalmitis after cataract surgery: making the most of the evidence. Ophthalmology 2007; 114: 831-832.
-
Riss B, Riss P. Corneal sensitivity in pregnancy. Ophthalmologica 1981; 183: 57-62.
-
Weinreb RN, Lu A, Beeson C. Maternal corneal thickness during pregnancy. Am J Ophthalmol 1988; 105: 258-260.
-
Park SB, Lindahl KJ, Temnycky GO, Aquavella JV. The effect of pregnancy on the corneal curvature. CLAO J 1992; 18: 256-259.
-
Schultz KL, Birnbaum AD, Goldstein DA. Ocular disease in pregnancy. Curr Opin Ophthalmol 2005; 16: 308-314.
-
Saito Y, Tano Y. Retinal pigment epithelial lesions associated with choroidal ischemia in preeclampsia. Retina 1998; 18: 103-108.
-
Rahman J, Rahman W. Temporary blindness as a complication of eclampsia: observations on three cases. J Obstet Gynaecol 2002; 22: 87-88.
-
Abad FJ, Pons J, Micó M, Casterá DE, Bellés MD, Sánchez A. Categorías de riesgo de los medicamentos utilizados durante el embarazo: Guía rápida de consulta. FAP 2005; 3: 49-61.
-
Vademecum Internacional. 50ª edición; Madrid: CMP Medicom; 2009.
-
Cortés C, Cuesta T, Gil MR. Fármacos midriáticos: pautas de dilatación en cirugía de cristalino. En: Lorente R, Mendicute J. LXXXIV Ponencia Oficial de la Sociedad Española de Oftalmología. Cirugía del Cristalino. Madrid: Industria gráfica MAC LINE SL; 2008; I: 421-427.
-
Loukovaara S, Immonen I, Teramo KA, Kaaja R. Progression of retinopathy during pregnancy in type 1 diabetic women treated with insulin lispro. Diabetes Care 2003; 26: 1193-1198.
-
Gilbert-Barness E, Drut RM. Association of sympathomimetic drugs with malformations. Vet Hum Toxicol 2000; 42: 168-171.
-
Encinas JL, Cajigal C. Midriáticos. Colorantes. En: García-Sánchez J, García-Feijoó J. Oftalmomecum 2009. Barcelona: P. Permanyer; 2009: 11-18.
-
Halperin LS, Olk RJ, Soubrane G, Coscas G. Safety of fluorescein angiography during pregnancy. Am J Ophthalmol 1990; 109: 563-566.
-
Arias A, García MC, Matilla M. Antiinfecciosos. Antimicrobianos, antifúngicos y antivirales. En: García-Sánchez J, García-Feijoó J. Oftalmomecum 2009. Barcelona: P. Permanyer; 2009: 19-50.
-
De las Heras B, Arias A, Bañuelos J, García C. Antibióticos. En: Cortés C, Arias A, Encinas JL, García-Feijoó J. LXXXIII Ponencia Oficial de la Sociedad Española de Oftalmología. Farmacología Ocular. Madrid: Industria Gráfica MAE SL; 2007: 77-108.