REQUESTED PUBLICATION
Infections in corneal refractive surgery with excimer laser
DE ROJAS V1, LLOVET F2, MARTÍNEZ M3, COBO-SORIANO R2, ORTEGA-USOBIAGA J4, BELTRÁN J5, BAVIERA J5
1 Ph.D.
in Medicine and Surgery. Ophthalmology Specialist. Baviera Clinic - European
Ophthalmological Institute (A Coruña).
2 Ph.D. in Medicine and Surgery. Ophthalmology Specialist. Baviera
Clinic - European Ophthalmological Institute (Madrid).
3 Graduate in Medicine and Surgery. Ophthalmology Specialist. Baviera
Clinic - European Ophthalmological Institute (Madrid).
4 Ph.D. in Medicine and Surgery. Ophthalmology Specialist. Baviera
Clinic - European Ophthalmological Institute (Bilbao).
5 Graduate in Medicine and Surgery. Ophthalmology Specialist. Baviera
Clinic - European Ophthalmological Institute (Valencia).
ABSTRACT
Corneal refractive surgery with excimer laser has become the choice procedure for correcting ametropia. LASIK is at present the preferred technique due to the quick visual recovery and the low rate of complication. However, the popularity of surface ablation procedures are on the rise due to their lower susceptibility to complications related to the lenticle and a lower risk of ectasia. In addition, their efficiency and safety are well documented. However, any of these techniques raises concerns about the development of infectious keratitis which, although rate, is a potentially severe complication. This review analyzes the prevalence, day of onset, result of cultures, risk factors, presentation symptoms, diagnostic, treatment and final visual acuity in infections after LASIK and surface ablation procedures. The prevalence of post-LASIK infection is low, but the infection rate after superficial ablation is statistically higher. The most frequently involved germs are gram-positive, probably from the ocular surface flora. However, atypical germs cannot be discarded and a microbiological diagnostic is required, even more so in LASIK cases. As this is a complication with potentially severe visual consequences, it is recommended to establish a post-op prophylactic topical treatment, with fourth generation fluoroquinolones being the medication of choice, particularly moxifloxacin. The onset of this complication in asymptomatic patients underscores the need of an adequate post-op assessment program. It is recommended to initiate aggressive early treatment of the infection with fortified antibiotics after raising the lenticle and antibiotic cleansing of the interfase in the case of LASIK. The early raising of the lenticle is associated to a tendency to improved visual results, lower leukoma rates and lower number of subsequent visual rehabilitation procedures. An adequate early treatment can achieve satisfactory visual results. We have not found statistically significant differences between the final visual acuity after infections post-surface ablation and LASIK even though the treatment of infections after LASIK is more complex. In what concerns post-LASIK microbacterial infections, their treatment is complex due to delayed diagnostics, resistance to conventional antibiotics, slow response to treatment, inadequate penetration of the antibiotics and resistance to monotherapy.
Key words: LASIK, surface ablation, refractive photokeratectomy, LASEK, Epi-LASIK, infectious keratitis.
INTRODUCTION
Corneal refractive surgery with excimer laser has become the first choice procedure for correcting ametropia. At present, LASIK is the preferred procedure due to the quick visual rehabilitation and the low rate of complications (1,2). However, post-LASIK infectious keratitis is a severe complication which involves a risk of visual acuity loss.
Surface ablations include refractive photokeratectomy (PRK), LASEK and Epi-LASIK. The popularity of this type of procedures is rising due to their lower susceptibility of complications related to the lenticle, lower ectasia risk and well-documented efficacy and safety (3-10). However, the visual recovery time is longer and there is concern about the development of infectious keratitis which, although rare, is a potentially severe complication.
The objective of this review is to analyze the prevalence, day of onset, culture results, risk factors, presentation symptoms, diagnostic, treatment and final visual acuity in infections after LASIK and surface ablation procedures. Due to their peculiar characteristics, post-LASIK microbacterial infections shall be discussed in a separate section.
PREVALENCE
The prevalence of this type of infections is difficult to determine. Being rare, long series of patients are needed to calculate said prevalence. Until very recently, most publications include series with limited sample numbers.
LASIK
Two retrospective series report a prevalence of 2 cases in 1062 eyes (2) and 1 case in 1019 procedures (1). The small number of cases does not allow an integrated analysis of data to draw useful conclusions for diagnostic and treatment. Two of the most important series comprise 15 and 17 cases respectively referred from various centers to third level reference institutions, making it impossible to calculate prevalence (11,12).
A review of the literature made in 2004 concluded that prevalence is variable (0.02%-1.5%) (13). Said review included 103 infections of 87 patients in 42 analyzed articles. However, many infection cases may not be published and said numbers could underestimate the actual rate of infections. In addition, frequently the worst cases are published, thus biasing the end results for visual acuity and sequels.
The American Society of Cataract and Refractive Surgery (ASCRS) has carried out surveys to obtain data on prevalence and the most frequent causes of infection. On the basis of said surveys, the estimated prevalence was of 1 case in 2,919 procedures (14) and 1 case in 2,131 procedures (15). However, these estimates are subject to a «no reply» bias in surveys with a response rate under 66%.
As the post-LASIK infection rate is low, the analysis of numerous series of cases from a single centre could reveal additional data about certain clinical parameters and enable a better understanding of the presentation, etiology and treatment of these infections. However, due to the low prevalence of this complication, many patients would be needed to draw useful conclusions and such a high number of cases is difficult to find in a single institution. Two series of this type have been published. One found 10 cases of infection in 10,477 eyes (16) and the other, with the highest number of cases to date, found 72 cases in 204,586 procedures (0.035%) (17) (table 1). In the latter series, Llovet et al (17) made a retrospective analysis of the post-LASIK infections detected in all the centers of our institution.
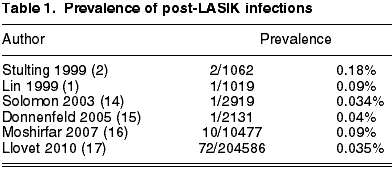
This series summarizes the advantages of the two types of previous studies. On the one hand, it is based on a population sufficiently numerous to exactly estimate the prevalence of an infrequent complication. This fact also facilitates the study of the infection in a situation in which most of the involved variables are controlled because, even though the patients were intervened in different centers of the same institution, the pre-op, intra-op and post-op protocols followed by patients and surgeons are the same. On the other hand, a sample of infection cases is sufficiently numerous to draw conclusions about the results of cultures, risk factors, treatment and visual results.
Surface ablation
Wroblewski (18) and Leccisotti (19) found a prevalence of five cases in 25,337 PRK procedures (0.019%) and 2 cases in 10,452 procedures (0.02%) respectively, whereas Machat (20) and Oliveira (21) estimated a prevalence of keratitis after PRK of 1/1000 (0.1%) and 9/4492 (0.2%), respectively.
As in the LASIK infections, the small number of cases makes it difficult to carry out an integrated analysis and to draw conclusions about diagnostic and treatment. Two numerous series include 13 and 16 cases respectively after PRK, but do not specify the total number of procedures, and thus it is not possible to calculate prevalence. The first series is a 2003 review of literature comprising 26 cases after PRK (22), and the other series only provides information about the result of cultures (23).
In what concerns LASEK and Epi-LASIK, the only publications of cases were only symbolic: 5 cases after LASEK (24-27) and 2 cases after Epi-LASIK (28). The prevalence of infections after these procedures could be similar to that of PRK, as they include the same infection risk factors.
Recently, we have presented the most numerous series of cases after surface surgery, comprising 39 cases of 38 patients in 18,651 procedures, giving a prevalence of 0.2%, or 1 case out of 500 procedures. If we take into account only the cases with positive culture (13 cases), the prevalence of proven infections would be of 0.06%.
As it was a retrospective study which excluded cases compatible with sterile infiltrations, it is possible that some of the cases with negative culture did not have an infectious etiology (de Rojas V, Llovet F, Martínez M, Beltrán J, Baviera J. Infectious keratitis in 18,651 laser surface ablation procedures. XXVIII Congress of the ESCRS. Paris, 4-8/IX/2010). The total prevalence of infections is similar to that published by Machat (20) and de Oliveira (21), but 10 times greater than that estimated by Wroblewski (18) or Leccisotti (19). The reason for a lower rate of infections in the series of Wroblewski and Leccisotti is not clear. Both are retrospective studies, and may not have collected some cases of infection. Particularly, the Wroblewski series included the cases of infection after surface surgery in six centers of the US Navy, and it is possible, they underestimated the prevalence if the patient was destined somewhere else. On the other hand, our rate of infections after LASIK is one of the lowest in the literature. As the institution, the protocols and the study period is the same, our rate of infection after surface surgery should not be greater than that of other centers. This also suggests that the series exhibiting lower infection rates after surface surgery could be underestimating prevalence.
All the series mentioned above include patients treated with PRK, while our series includes patients treated with PRK or LASEK. Few cases of infection after LASEK have been published (24,27). However, we assume that the risk of infection should be similar to that of PRK, and therefore we have considered the prevalence and presentation characteristics of both procedures together (table 2).
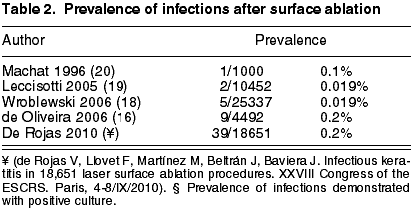
Accordingly, it seems that the prevalence of infection after surface procedures could be greater than the prevalence after LASIK procedures. This is not surprising for three reasons. Firstly, there is an epithelial defect which takes about four days to close; second, the use of a prolonged use contact lens increases the risk of infectious keratitis; third, the use of topical corticoids for controlling the tissue repair response could suppress the immune system ability to fight against the infection. If we take into account that in the same institution the infection rate after LASIK is of 0.035% (17) and that the rate of infection after the surface ablation is of 0.2% for total infections and 0.06% for demonstrated infections (Rojas V, Llovet F, Martínez M, Beltrán J, Baviera, J. Infectious keratitis in 18,651 laser surface ablation procedures. XXVIII Congress of the ESCRS. Paris, 4-8/IX/2010), this would be of 5.7 to 2 times greater (p<0.001 or p<0.05 test c2). As the institution, the protocols, surgery rules and surgeons are the same, the only factor that could explain the difference in the infection rate is the type of surgery in itself. This finding is in agreement with the results of a previous study in which the infection rate after LASIK was of 0.1% against 0.2% after PRK (21). The continued analysis of the relative safety and efficacy of these procedures is very important in what concerns the informed consent and evidence-based medicine.
INFECTION PRESENTATION TIME AFTER SURGERY
LASIK
The infections after LASIK had been classified as early if they appear in week one after LASIK and late if appearing later (13). This classification is useful for treatment as the symptom onset time varies depending on the microorganism that is causing the infection. In the literature review by Chang, 49.4% of infections were early and 50.6% late.
Infections due to gram positive bacteria tend to appear within the first week after LASIK, while the infections by nontuberculous mycobacteria occur with greater frequency after the first week (13). In our series, the time elapsed from surgery up to the onset of symptoms was of 16±31 days (range 1-180), being early in 62. 5% of cases (17). No grouping of cases was detected.
Surface ablation
The time elapsed between surgery and the appearance of the first symptoms in surface surgery is under one week in the majority of cases (18,19,21,22). In our series, 71.79% of cases emerged in the first week, which is a higher percentage than for the LASIK cases (de Rojas V, Llovet F, Martínez M, Beltrán J, Baviera J. Infectious keratitis in 18,651 laser surface ablation procedures. XXVIII Congress of the ESCRS. Paris, 4-8/IX/2010).
RESULT OF CULTURES
LASIK
According to a survey by the ASCRS, the organisms most frequently involved in post-LASIK infections during 2001 were the nontuberculous mycobacteria (28% of cases) followed by staphylococci (20%) (14). Another survey made in 2004 presented at the 2005 ASCRS demonstrated a reduction in the number of nontuberculous mycobacteria with only two reported cases. 61% of the cases reported in this survey were caused by staphylococci (15).
Review of the literature about published infectious keratitis cases after LASIK, of about 100 cases with culture results 47% had been caused by nontuberculous mycobacteria and 19% by staphylococci (13). In the series of 10 cases published by Moshirfar, the most frequent etiology was also nontuberculous mycobacteria followed by staphylococci (16).
All the microorganisms found in our series were gram positive (21 cases out of 54 in which samples were taken; the rest gave a negative result). The isolated bacteria were: Staphylococcus epidermidis (9 cases), Streptococcus pneumoniae (8 cases), Streptococcus viridans (2 cases), Streptococcus pyogenes (1 case) and Staphylococcus aureus (1 case). No case of polymicrobial infection or caused by mycobacteria or fungi was detected, but this cannot be discarded in a situation with a high rate of negative cultures. However, the fungal etiology does not seem probable because none of the cases required antifungal therapy. The onset of the symptoms was early in all positive culture cases except in one in which de-epithelialization occurred during surgery with subsequent relapsing erosions. All the cases with negative culture exhibited late presentation (17). Garg et al (12) recently published a series of 17 cases of 15 patients. The isolated microorganisms were fungi (4 eyes), Nocardia asteroides (5 eyes), nontuberculous mycobacteria (4 eyes), Acanthamoeba (2 eyes), Corynebacterium (1 eye), Staphylococcus epidermidis (1 eye). Nocardia, fungi and Acanthamoeba are rare causes for infection after LASIK. A detailed analysis of these cases reveals that the publications about these infrequent microorganisms are from countries with tropical climates. It is also possible that, as these studies proceed from the third level centers, they may select the worst infections, i.e., those which do not respond to standard broad spectrum range tend to be referred to reference centers (12). The etiology for gram negative terms is rare in all series (11,16,29,30).
The above results demonstrate that although some studies (13,14,16) have found that nontuberculous mycobacteria were the most frequent cause of infections after LASIK, the most recent studies have detected increases in infections caused by staphylococci (15) and methicillin-resistant bacteria after LASIK (Kim TK. Cornea Day. ASCRS Congress. Chicago, 4-9/IV/2008). This change could reflect the growing awareness about the need of performing LASIK in adequate aseptic conditions (31,32) (that would explain the reduction of infections caused by mycobacteria) in which case the ocular flora of the patient would become the main source of microorganisms causing infections, as occurs in other infections after ophthalmological procedures. The increase of infections caused by methicillin-resistant S aureus (MRSA) could evidence of the increased rate of MRSA carriers in the community, as recently documented (33). A similar trend towards an increased number of infections caused by MRSA has been indicated by Deramo et al in post-op endophthalmitis (34). And, as will be stated in the risk factors section, health workers are at greater risk of infection by meticillin-resistant staphylococci (35).
Surface ablation
In our analysis of infectious keratitis after surface ablations, we detected 39 cases of infection in 18,651 procedures. Samples were taken in 27 cases of which 13 were positive for gram positive bacteria: Staphylococcus species (9 cases: Staphylococcus epidermidis, 1 case; Staphylococcus aureus, 4 cases; Staphylococcus species, 4 cases), Pseudomonas species (1 case) and Streptococcus pneumoniae (2 cases). Candida parasilopsis was identified in one contact lens case, but not in the corneal sample –possible contamination– (de Rojas V, Llovet F, Martínez M, Beltrán J, Baviera J. Infectious keratitis in 18,651 laser surface ablation procedures. XXVIII Congress of the ESCRS. Paris, 4-8/IX/2010). Wroblewski detected four cases of S. aureus including two methicillin-resistant cases (18). Out of 16 cases of infection after refractive keratectomy published by Leal, all were caused by gram positive microorganisms excepting one case, produced by Penicillium (23). In the series of 13 eyes with infection after PRK published by Donnenfeld (22), all the bacteria involved were gram positive, mostly staphylococci. The same authors made a review of cases published to that date in the literature and found the following causal agents: Mycobacterium chelonee (4), triple fungal etiology (Acremonium, Penicilium, Aureobasidium), Scopulariopsis and Aspergillus. Of the 10 remaining cases, four were staphylococci species, two were streptococci species, and one case of Pseudomonas aeruginosa.
Even though previous studies on infection and related contact lenses have documented a high prevalence of infections due to Pseudomones aeruginosa (36), our series has only one case, and a review of the literature includes one more (22) in a patient that did not receive prophylactic antibiotic treatment. In addition, the contamination of contact lenses after three days in patients treated with PRK and under prophylaxis with topical antibiotics was evaluated. 7.4% were positive for gram positive bacillum and in only one case gram negative bacteria was isolated (1.2%) (37).
ORIGIN OF GERMS, RISK FACTORS AND PREVENTION
LASIK
The potential risk factors associated to LASIK keratitis published in the literature include blepharitis, dry eye, epithelial defects, therapeutic contact lenses and environmental factors (11,13,15,17,35).
Several possible sources of microorganisms have been considered, including surgery instruments, the surgeon’s hands, environmental factors and ocular and periocular surface flora. The contamination rate of the interface after LASIK has been assessed in a recent study at 24.5% (38). As in other intraocular surgery contamination studies (39), the most frequently isolated microorganism was staphylococci. This is not surprising as it is part of the normal conjunctival flora, and it is believed that the bacteria causing surgical complications are located in the eyelids and the conjunctiva. However, in 38.8% of contamination cases cultures of palpebral margins, conjunctiva and instruments gave negative results, and therefore the causes of contamination in these cases could not be determined.
The most frequently involved gram positive bacteria (S. aureus and coagulase-negative staphylococci) are generally associated to blepharitis. A careful exploration of the eyelids is recommended for detection and treatment before surgery with palpebral hygiene, oral tetracycline and topical antibiotics.
Exposure to the sanitary environment constitutes a risk factor for methicillin-resistant staphylococci infections, both in LASIK as in PRK, as demonstrated by the publication of testimonial cases (16,40,41) as well as a recent series (35). Solomon et al published a series of 13 eyes in 12 intervened patients, 10 of which were for LASIK and 2 for PRK. Nine of these patients were health workers or were exposed to the sanitary environment (35). Health workers have a greater prevalence of colonization by methicillin-resistant S. aureus than the general population. On the basis of these series, it is recommended to carry out pre-op prophylaxis on patients exposed to the health environment, with palpebral hygiene and bacitracin or fourth generation fluoroquinolones and take a nasal sample to detect carriers. It is also recommended to consider monocular treatment carrier patients (35).
Generally, infections due to fungi and mycobacteria are related to failures in sterilization measures and/or sterile intra-op techniques. Epidemic groupings of cases have been published, all of them caused by nontuberculous mycobacteria, in which the source of the infection was Mycobacterium chelonee from a contact lens utilized as a mask during the ablation (42); M. szulgai was identified in the ice utilized for calling the BSS in the surgical field (43); M. chelonee was isolated from the portable steam sterilizer utilized for sterilizing the microkeratome (44).
Factors such as systemic immunosuppression or diabetes do not seem to increase the risk of infection after LASIK (45).
Surface ablation
The potential risk factors for infections in surface ablations reported in the literature include blepharitis, manipulation of the contact lens (22) and the sanitary environment (35).
Prophylactic antibiotic treatment
As discussed above, at present the greatest risk is posed by gram positive microorganisms, and due to the risk factors associated with infectious keratitis, it is recommended to utilize prophylactic antibiotics after surface ablation. Prophylactic treatment with broad range antibiotics has considerably diminished the number of cases with positive culture in an interphase contamination model in pigs (46). A recent study (47) has analyzed the susceptibility to antibiotics of bacteria isolated from patients who were to be intervened for refractive surgery. The isolated microorganisms were the following: 85% staphylococci coagulase-negative, 2.3% S. aureus, 1.2% S. pneumoniae and 4.8% gram negative bacillus. The most effective antibiotics against these bacteria were gemifloxacin, moxifloxacin and gatifloxacin.
Accordingly, the antimicrobials of choice are fourth-generation fluoroquinolones as they provide a broad range of coverage, both for gram positive and gram negative bacteria (48) with low toxicity and good ocular penetration (49), particularly moxifloxacin. Fourth-generation fluoroquinolones a more efficient against microorganisms that are resistant to previous fluoroquinolones (50), exhibit greater activity against gram positive bacteria, particularly S. pneumoniae (48), as well as greater activity against anaerobic bacteria (48) and nontuberculous mycobacteria (51,52).
However, fluoroquinolones exhibit two weak points in their coverage. First, even though they have greater activity against MRSA than the previous fluoroquinolones (50), their efficacy against MRSA is lower than against S. aureus methicillin-sensitive (MSSA) (53). Second, they can be less active against Pseudomonas aeruginosa than cyprofloxacin and do not cover Pseudomonas aeruginosa cyprofloxacín-resistant (53). In fact, a smaller number of cases have been published describing clinical resistance in these two situations (54).
PRESENTATION SYMPTOMS
LASIK
In our series, symptoms were detected in 54% of patients, with nine cases being asymptomatic (17). In these cases, the infection was diagnosed in one of the routine post-op visits, which evidence is the need of an adequate follow up of patients. As 62.5% of cases appeared during the first week and 90.27% during the first month, recommended schedule of post-op visits would be as follows: One day, seven days, one month and three months. 41.6% of symptomatic eyes exhibited pain, 34.72% exhibited visual acuity reduction and 55.5% red eye. In turn, 9.7% of patients experienced photophobia and 28.83% complained of epiphora. These percentages are similar to those published in a review (13).
Surface ablation
In what concerns the surface ablation cases, 11 eyes of 39 cases with infection were asymptomatic. 71.79% of cases emerged in the first week and 89.74% in the first month. This underscores the need of carrying out routine visits at the appropriate time. The eyes with symptoms exhibited pain (48.71%), and diminished visual acuity (69.23%), red eye (46.15%), photophobia (17.94%), epiphora (25.64%) and discomfort (56.41%) (de Rojas V, Llovet F, Martínez M, Beltrán J, Baviera J. Infectious keratitis in 18,651 laser surface ablation procedures. XXVIII Congress of the ESCRS. Paris, 4-8/IX/2010).
DIAGNOSTIC
Clinical diagnostic
LASIK
The main sign of infection after LASIK is the presence of focal infiltrates in the interphase (13,17). These infiltrates must be differentiated from other causes of keratitis after LASIK, particularly diffuse lamellar keratitis, but also from sterile peripheral infiltrates or multifocal lamellar keratitis (16,55). Infections in the interphase could initially be confused with diffuse lamellar keratitis, which delays the establishment of the appropriate treatment (56). The differential diagnostic between diffuse lamellar keratitis and infectious keratitis is very important as both conditions require different treatments. In diffuse lamellar keratitis the infiltrate is diffuse, without predominant foci and is limited to the interphase without extending to the stromal bed or the lenticle. In contrast with infectious keratitis, there is no reaction in the anterior chamber or pain. However, it must be noted that diffuse lamellar keratitis could be associated to an infectious infiltrate and it exhibits a non-specific reaction to any inflammatory stimuli in a cornea with lamellar structure.
Surface ablation
Surface ablation is a similar case. The main infection sign includes the presence of infiltrates, which, in the case of surface ablation, must be differentiated from sterile infiltrates (related to the use of contact lenses or topical nonsteroid anti-inflammatories). The latter are localized in the periphery or the mean territory, have a small size (1-3 mm) and exhibit no reaction in the anterior chamber. In the majority of cases, the epithelium is intact or exhibits slight keratopathy and the infiltrates can be located beyond the epithelial defect area induced by surgery (18,57).
Microbiological diagnostic
LASIK
Sample taking for culture is essential in any type of infection after surface surgery, but particularly after LASIK. The microorganisms involved could be atypical, difficult to predict and may not respond adequately to empirical treatment. In addition, the majority of infections after LASIK exhibit multifocal stromal infiltrates which sometimes do not exhibit typical characteristics such as feathery edges even if the etiology is fungal (12). The collection of samples for culture provides other advantages in addition to the microbiological diagnostic, such as:
a) removal of infected tissue.
b) enhanced penetration of antibiotics.
The ASCRS White Paper on LASIK infections recommends to take culture in agar blood, agar chocolate, Sabouraud thioglycolate and thioglycolate broth. If the infection emerges over the two weeks after LASIK, it is recommended to add means for growth of nontuberculous mycobacteria –Lowenstein-Jensen or Middlebrook 7H-9– in addition to the others, because this type of germ tends to appear later. If the sample is sufficient, it is also recommended to perform extensions for staining with Gram, methenamin silver and Ziehl-Neelsen or auramine/rhodamine to discard infrequent pathogens such as Nocardia, non-tuberculosis mycobacteria and fungi. If the cultures were negative and the infection worsens, a corneal biopsy or PCR must be considered (15).
If the material is scarce, an extension should be taken. In addition to an enriched culture medium (thioglycolate or brain-heart infusion broth) and, whenever possible, a further extension or an agar (23).
Surface ablations
As discussed above, most of the infections after a surface surgery are caused by gram positive bacteria. Even so, the literature includes published cases caused by fungi and mycobacteria, although with less frequency than in LASIK surgery. For this reason, it is convenient to include specific plates for these microorganisms. In the case of surface ablation, it is recommended to also carry out a culture of the contact lens in thioglycolate broth (21).
TREATMENT
LASIK
The treatment of post-LASIK infections is not simple. Atypical microorganisms under a lenticle constitute a diagnostic and therapeutic challenge. The localization in the interphase hinders access for taking samples and does not allow adequate penetration of antibiotics. Empirical treatment without previous samples is not recommended as some bacteria involved do not respond to conventional treatment. For this reason, an aggressive therapeutic approach is recommended with immediate lenticle raising, sample taking for culture and extensions and interphase wash with antibiotics (fortified vancomycin 50 mg/ml for early onset infections and fortified amikacin 35 mg/ml for late onset infections) (15). Treatment with initial raising of the lenticle is associated to better visual results, less residual leukoma and lower number of visual rehabilitation procedures than the initial treatment with topical antibiotics (13) (Llovet F, de Rojas V, Martínez M, Baviera J. Infectious keratitis after LASIK: Therapeutic approach. 25 Congress of SECOIR. Cadiz, 19-22/V//2010). In addition, in our series 10 out of the 18 cases initially treated with topical treatment without interphase irrigation subsequently required lenticle raising and antibiotic wash (17).
The treatment is initiated later in empirical manner as per the patterns described below and/or guided by the stain results of the extensions if it was possible to carry them out.
The treatment is modified on the basis of the Clinical response and the culture results. The ASCRS White paper recommends different combinations depending on whether the infection appeared early or late (fig. 1). This treatment does not cover fungal infections and therefore the result must be subsequently modified according to the culture results (15).

Fig. 1. Treatment recommendations for infections after LASIK according to the
ASCRS White Paper (15).
It is recommended to add oral doxicyclin (100 mg twice a day) as it inhibits collagenase production and suspend topical corticoids.
Amputation of the flap
Even when the treatment was adequate, on some occasions it is necessary to amputate the lenticle because, on the one hand, it does not allow an adequate penetration of antibiotics, and, once it becomes necrotic, it loses all its optical qualities and also becomes a refuge for microorganisms, which hinders the success of the treatment. Lenticle amputation is not infrequent in infections after LASIK (13,15), particularly when atypical or aggressive bacteria are involved. In a literature review of 103 infections, 37 lenticles were amputated (13), mostly in infections caused by aggressive mycobacteria or microorganisms. The series referring to epidemic groupings of nontuberculous mycobacteria demonstrated that nearly 50% of affected eyes required lenticle amputation (42-44). In the series of Karp (11), 5 out of 15 eyes with post-LASIK infections –all caused by nontuberculous mycobacteria– required lenticle amputation. The publications by Moshirfar et al (16,54) describe one case of keratitis caused by P. aeruginosa out of a total of 10 eyes with infectious keratitis that required lenticle amputation (16) as well as one case of infection by MRSA (54). The study by Solomon et al (35) referred that two lenticles had to be amputated out of 12 cases of MRSA infections, and in our study the case that required amputation was caused by S. pneumoniae (17).
In the situations in which it is necessary to amputate the flap, the removed corneal tissue must be sent for anatomopathologic analysis with adequate staining (hematoxilin-eosin, PAS, Ziehl-Neelsen) in order to identify the microorganism if it could not be isolated previously.
Keratoplasties
Between 10% (58) and 14.5% (13) of infections after LASIK require surgical keratoplasty treatment.
Enucleation
The literature described three cases that required enucleation, one in the ASCRS survey (14) and two in the Karp et al series (11), one caused by Acanthamoeba and the other by Fusarium oxysporum.
Surface ablation
In surface infections, after removal of the contact lens and the taking of samples for culture, it is recommended to begin topical treatment with a combination of broad range antibiotics, taking into account that the most frequent causative organisms are gram positive. The same pattern discussed in the previous section can be recommended for early starting infections. In addition, it is convenient to add oral doxicyclin (100 mg/every 12 hours) to inhibit the collagenases.
Keratoplasties
In our series, the 39 cases of infection were resolved with medical treatment, but one case required keratoplasty for visual rehabilitation. One out of 13 cases (22) and one out of two cases (19) also required keratoplasty in two previous series.
VISUAL RESULTS
LASIK
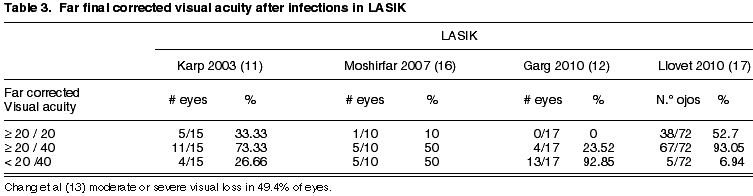
The final visual acuity after treatment of post-LASIK infections varies between series (table 3). Our series (17) demonstrate better results than those published previously (11,13,16). Several factors could explain this difference: firstly, the results by Karp et al (11), Garg et al (12) and Chang et al (13), derived from the analysis of publications or series of cases referred to third level centers respectively, which means that the worst cases could have been selected, thus biasing the results. Secondly, in our study we did not detect cases of fungal or mycobacteria infections, which have a considerably worse prognosis than those caused by gram positive bacteria (11-13). Our results are also better than those of Moshirfar (16). This group reported results in which only 30% of eyes reached a visual acuity of 20/40 and only 20% of the eyes achieved 20/20. Continuing with this series, 4 out of 10 cases were caused by mycobacteria and in addition two polymicrobial infections occurred, one caused by mycobacteria and the other by Alternaria (16).
Surface ablation
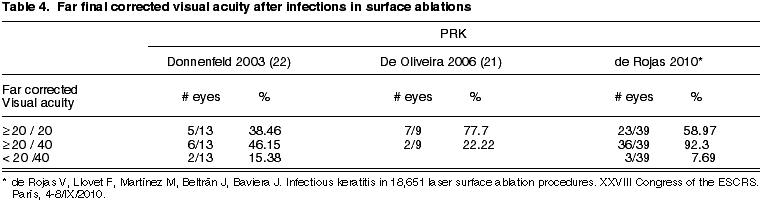
The final visual results of our series of infections in surface ablation are equal to those published by other authors or slightly better (table 4).
In what concerns visual results, no statistically significant differences were found between the fine and visual acuity after infections post- surface ablation (de Rojas V, Llovet F, Martínez M, Beltrán J, Baviera J. Infectious keratitis in 18,651 laser surface ablation procedures. XXVIII Congress of the ESCRS. Paris, 4-8/IX/2010) and after LASIK (17) (p=0.901; test Mann-Whitney) (fig. 2), even though the treatment of the post-LASIK infections is more complex.
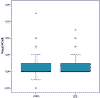
Fig. 2. Comparison of final corrected visual acuity (FCVA) (log MAR) after
infectiosn in LASIK (17) and surface surgery (SS). (de Rojas V, Llovet F,
Martínez M, Beltrán J, Baviera J. Infectious keratitis in 18,651 laser surface
ablation procedures. XXVIII Congress of the ESCRS. Paris, 4-8/IX/2010).
KERATITIS DUE TO NONTUBERCULOUS MYCOBACTERIA AFTER LASIK
The infections after LASIK caused by nontuberculous mycobacteria merit special attention. As will be seen below, Treatment is complex, due to delays in diagnostic, resistance to conventional antibiotics, slow response to treatment, inadequate penetration of antibiotics and resistance to monotherapy (58).
Even though at present the tendency has been identified towards a gram positive bacteria as the main cause of infections in LASIK (15,17), previous studies have found that nontuberculous mycobacteria were at one time the most frequently involved microorganisms in LASIK infections (13,14,16,58).
Nontuberculous mycobacteria are ubiquitous microorganisms that are found on the floor, water and food (59) and intra-op contamination has been proposed as the most probable source of infections in LASIK (60,61). At least four epidemic outbreaks of mycobacteria infections after LASIK (15,42-44) have been described, which demonstrates the importance of adequate sterilization of surgical instruments and the necessity of utilizing intra-op sterile techniques as well as performing the surgery in the operating room. It is possible that increased awareness about these points may play a role in the reduction of infections derived from this etiology observed in recent studies.
A review of the 2005 literature (58) identified 50 cases confirmed by culture of infections caused by mycobacteria (66% Mycobacterium chelonee, 14% Mycobacterium abscesos, 10% Mycobacterium szulgai; 4% Mycobacterium mucogenicum; 4% Mycobacterium fortuitum and 2% Mycobaterium terrae). The time of onset of symptoms varied between three days to six months. The mean time of appearance of infections by the slow-growing M. szulgai was of 2.5 months, whereas that of fast-growing bacteria was of 3.4 weeks. The mean expression time of all mycobacteria was greater than that of presentation of infections caused by fungi or other bacteria (11-13). The mean time up to onset was of 21.17 days –from 10 to 65 days–. The clinical course is more latent and less symptomatic than infections caused by other bacteria, which leads to a later diagnostic and to occasionally confusing them with diffuse lamellar keratitis (58). Samples for culture and extensions in adequate media, and with specific staining is required for microbiological diagnostic. The adequate stains are Ziehl-Neelsen or fluorochromic stains (auramin, rhodamin) and the Löwenstein-Jensen or Middlebrook media. The PCR is not routinely carried out, but it can be considered when suspected with negative cultures, while electrophoresis can be utilized in epidemic outbreaks to compare isolated microorganisms and study the origin of the contamination (42,43).
Keratitis due to mycobacteria is more difficult to treat than other types of bacteria and keratitis, and it frequently requires combined treatment. In the past, amikacin was considered the treatment of choice for Mycobacterium keratitis (62). However, Amikacin had a high failure rate in treatments (60%) (63) due to its poor penetration through the intact epithelium (64). Amikacin has been frequently utilized in combination with clarithromycin, but its use is limited by its toxicity and poor tolerance (63). Topical azithromycin has also been utilized (42). Cyprofloxacin has been utilized as an alternative or in combination with fortified antibiotics and constituted a first line treatment for several corneal infections, although resistances have become a limiting factor (65). Fourth-generation fluoroquinolones, particularly moxifloxacin, have significant advantages over fluorquinolones of previous generations for treating mycobacteria infections, including superior anti-bacterial activity, higher corner concentration and a reduced resistance rate (66-68), in addition to having synergic action with clarithromycin (69).
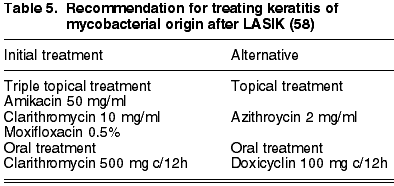
however, it is convenient to take into account that indications of resistance have arisen in clinics and laboratories to some strands of fourth-generation fluoroquinolones (70-72). Corticoids must be suspended during an active infection as they prolong and worsen the course of the infection (63). Considering the above, the majority of nontuberculous mycobacteria infection cases (49%) required treatment with several antibiotics concurrently (58) and it is recommended to begin treatment with a triple therapy as indicated in table 5, adding treatment with oral antibiotics (clarithromycin or doxicyclin). The time from the diagnostic to resolution of symptoms is extended, having a mean of 71.5 days. The literature review made by John referred that 54% of cases required lenticle amputation and four required keratoplasty (58).
CONCLUSIONS
Infections after LASIK are rare, but infections after surface ablation are statistically more frequent. The most commonly involved microorganisms are gram positive, probably originating in the ocular surface flora. However, atypical microorganisms cannot be discarded, making it essential to obtain a microbiological diagnostic, above all in LASIK cases. As this is a complication with potentially severe visual consequences, prophylactic topical treatment is recommended in the post-op period, with fourth-generation fluoroquinolones and particularly moxifloxacin being the agent of choice. The appearance of this complication in asymptomatic patients underlines the necessity of an adequate post-op follow up program. It is recommended to establish aggressive and early treatment of the infection with fortified antibiotics. After raising the lenticle and, in the case of LASIK, carrying out an antibiotic wash of the interphase. The early raising of the lenticle in LASIK cases is associated to a tendency towards better visual result, lower leukoma rate and lower number of subsequent visual rehabilitation procedures. With early and adequate treatment it will be possible to obtain satisfactory visual results.
REFERENCES
-
Lin RT, Maloney RK. Flap complications associated with lamellar refractive surgery. Am J Ophthalmol 1999; 127: 129-136.
-
Stulting RD, Carr JD, Thomppson KP, Waring GO 3rd, Wiley WM, Walker JG. Complications of laser in situ keratomileusis for the correction of myopia. Ophthalmology 1999; 106: 13-20.
-
Randleman J, Woodward M, Lynn M, Stulting RD. Risk assessment for ectasia after corneal refractive surgery. Ophthalmology 2008; 15: 37-50.
-
Teus MA, de Benito-Llopis L, García-González M. Comparison of visual results between laser-assisted subepithelial keratectomy and epipolis laser in situ keratomileusis to correct myopia and myopic astigmatism. Am J Ophthalmol 2008; 46: 357-362.
-
Hondur A, Bilgihan K, Hasanreisoglu B. A prospective bilateral comparison of epi-LASIK and LASEK for myopia. J Refract Surg 2008; 24: 928-934.
-
Tobaigy FM, Ghanem RC, Sayegh RR, Hallak JA, Azar DT. A control-matched comparison of laser epithelial keratomileusis and laser in situ keratomileusis for low to moderate myopia. Am J Ophthalmol 2006; 142: 901-908.
-
Salz JJ, Maguen E, Nesburn AB, Warren C, Macy JI, Hofbauer JD, Papaioannou T, Berlin M. A two-year experience with excimer laser photorefractive keratectomy for myopia. Ophthalmology 1993; 100: 873-882.
-
Seiler T, Wollensak J. Myopic photorefractive keratectomy with the excimer laser. One-year follow-up. Ophthalmology 1991; 98: 1156-1163.
-
Wallau AD, Campos M. One-year outcomes of a bilateral randomised prospective clinical trial comparing PRK with mitomycin C and LASIK. Br J Ophthalmol 2009; 93: 1634-1638.
-
Randleman J, Loft E, Banning C Lynn MJ, Stulting RD. Outcomes of wavefront-optimized surface ablation Ophthalmology 2007;114; 983-988.
-
Karp CL, Tuli SS, Yoo SH, Vroman DT, Alfonso EC, Huang AH, Pflugfelder SC, Culbertson WW. Infectious keratitis afer LASIK. Ophthalmology 2003; 110: 503-510.
-
Garg P, Chaurasia S, Vaddavalli PK, Muralidhar R, Mittal V, Gopinathan U. Microbial keratitis after LASIK. J Refract Surg 2010; 26: 209-216.
-
Chang MA, Jain S, Azar DT. Infections following laser in situ keratomileusis: an integration of the published literature. Surv Ophthalmol 2004; 49: 269-280.
-
Solomon R, Donnenfeld ED, Azar Dt, Holland EJ, Palmon FR, Pflugfelder SC, Rubenstein JB. Infectious keratitis after laser in siu keratomileusis: results of an ASCRS survey. J Cataract Refract Surg 2003; 29: 2001-2006.
-
Donnenfeld ED, Kim TK, Holland EJ Azar DT, Palmon FR, Rubenstein JB, Daya S, Yoo SH; American Society of Cataract and Refractive Surgery Cornea Clinical Committee. ASCRS White Paper. Management of infectious keratitis following laser in situ keratomileusis. J Cataract Refract Surg 2005; 31: 2008-2011.
-
Moshirfar M, Welling JD, Feiz V, Holz H, Clinch TE. Infectious and non-infectious keratitis after laser in situ keratomileusis. Occurrence, management and visual outcomes. J Cataract Refract Surg 2007; 33: 474-483.
-
Llovet F, de Rojas V, Interlandi E, Martín C, Cobo-Soriano R, Ortega-Usobiaga J, Baviera J. Infectious keratitis in 204586 LASIK procedures. Ophthalmology 2010; 117: 232-238.
-
Wroblewski KJ, Pasternak JF, Bower KS, Schallhorn SC, Hubickey WJ, Harrison CE, Torres MF, Barnes SD. Infectious keratitis after photorefractive keratectomy in the United States Army and Navy. Ophthalmology 2006; 113: 520-525.
-
Leccisotti A, Bartolomei A, Greco G, Manetti C. Incidence of bacterial keratitis after photorefractive keratectomy [letter]. J Refract Surg 2005; 21: 95-96.
-
Machat JJ. Excimer laser refractive surgery: Practice and principles. Thorofare, NJ: SLACK Inc.; 1996: 359-400.
-
de Oliveira GC, Solari HP, Ciola FB, Lima AL, Campos MS. Corneal infiltrates after excimer laser photorefractive keratectomy and LASIK. J Refract Surg 2006; 22: 159-165.
-
Donnenfeld ED, O’Brien TP, Solomon R, Perry HD, Speaker MG, Wittpenn J. Infectious keratitis after photorefractive keratectomy. Ophthalmology 2003; 110: 743-747.
-
Leal F, Lima ALH, de Freitas D, Campos M. Análise laboratorial das ceratitis infecciosas secundárias à cirurgia refractiva. Arq Bras Oftalmol 2005; 68: 353-356.
-
Jung SW, Kwon YA, Lee MK, Song SW. Epidermophyton fungal keratitis following laser-assisted subepithelial keratectomy. J Cataract Refract Surg 2009; 35: 2157-2160.
-
Parthasarathy A, Theng J, Ti SE, Tan DT. Infectious keratitis after laser epithelial keratomileusis. J Refract Surg 2007; 23: 832-835.
-
Maverik KJ, Conners MS. Aureobasidium pullulans fungal keratitis following LASEK. J Refract Surg 2007; 23: 727-729.
-
Lifshitz T, Levy J, Klemperer I, Beer-Sheva I. Bacterial keratitis after laser subepithelial keratectomy. J Refract Surg 2005; 21: 94-96.
-
Nomi N, Morishige N, Yamada N, Chikama T, Nishida T. Two cases of methicillin-resistant Staphylococcus aureus keratitis after Epi-LASIK. Jpn J Ophthalmol 2008; 52: 440-443.
-
Sharma N, Sinha R, Singhvi A, Tandon R. Pseudomonas keratitis after laser in situ keratomileusis. J Cataract Refract Surg 2006; 32: 519-521.
-
Muñoz G, Alió JL, Pérez-Santonja JJ, Artola A, Abad JL. Ulcerative keratitis caused by Serratia marcescens after laser in situ keratomileusis. J Cataract Refract Surg 2004; 30: 507-512.
-
LaHaye LC, Rieke H, Farshad F. Cleaner LASIK is possible. J La State Med Soc 2007; 159: 30-36.
-
Kohnen T. Infections after corneal refractive surgery: can we do better? J Cataract Refract Surg 2002; 28: 569-570.
-
Grundmann H, Aires-de-Sousa M, Boyce J, Tiemersma E. Emergence and resurgence of methicillin-resistant Staphylococcus aureus as a public-health threat. Lancet 2006; 368: 874-885.
-
Deramo VA, Lai JC, Winokur J, Luchs J, Udell IJ. Visual outcome and bacterial sensitivity after methicillin-resistant Staphylococcus aureus-associated acute endophthalmitis. Am J Ophthalmol 2008; 145: 413-417.
-
Solomon R, Donnenfeld ED, Perry HD, Rubinfeld RS, Ehrenhaus M, Wittpenn JR Jr, Solomon KD, Manche EE, Moshirfar M, Matzkin DC, Mozayeni RM, Maloney RK. Methicillin-resistant Staphylococcus aureus infectious keratitis following refractive surgery. Am J Ophthalmol 2007; 143: 629-634.
-
Alfonso E, Mandelbaum S, Fox MJ, Forster RK. Ulcerative keratitis associated with contact lens wear. Am J Ophthalmol 1986; 101: 429-433.
-
Dantas PE, Nishiwaki-Dantas MC, Ojeda VH, Holzchuh N, Mimica LJ. Microbiological study of disposable soft contact lenses after photorefractive keratectomy. CLAO J 2000; 26: 26-29.
-
Feizi S, Jadidi K, Naderi M, Shahverdi S. Corneal interface contamination during laser in situ keratomileusis. J Cataract Refract Surg 2007; 33: 1734-1737.
-
Seal D, Reischl U, Behr A, Ferrer C, Alió J, Koerner RJ, Barry P; ESCRS Endophthalmitis Study Group. Laboratory diagnosis of endophthalmitis. Comparison of microbiology and molecular methods in the European Society of Cataract & Refractive Surgeons multicenter study and susceptibility testing. J Cataract Refract Surg 2008; 34: 1439-1450.
-
Rudd JC, Moshirfar M. Meticillin-resistant Staphylococcus aureus keratitis after laser in situ keratomileusis. J Cataract Refract Surg 2001; 27: 471-473.
-
Solomon R, Donnenfeld ED, Perry HD, Jensen HG, Stein J, Snyder RW, Wittpenn JR,Donnenfeld ED. Bilateral methicillin-resistant Staphylococcus aureus keratitis in a medical resident following uneventful photorefractive keratectomy. Eye Contact Lens 2003; 9: 187-189.
-
Chandra NS, Torres MF, Winthrop KL, Bruckner DA, Heidemann DG, Calvet HM, Yakrus M, Mondino BJ, Holland GN. Cluster of Mycobacterium chelonae keratitis cases following laser in-situ keratomileusis. Am J Ophthalmol 2001; 132: 819-830.
-
Fulcher SFA, Fader RC, Rosa RH, Holmes GP. Delayed-onset mycobacterial keratitis after LASIK. Cornea 2002; 21: 546-554.
-
Freitas D, Alvarenga L, Sampaio J, Mannis M, Sato E, Sousa L, Vieira L, Yu MC, Martins MC, Hoffling-Lima A, Belfort R Jr An outbreak of Mycobacterium chelonae infection after LASIK. Ophthalmology 2003; 110: 276-285.
-
Cobo-Soriano R, Beltrán J, Baviera J. LASIK outcomes in patients with underlying systemic contraindications: a preliminary study. Ophthalmology. 2006; 113: 1118-1124.
-
Wahab SA, Moreira H, Buquera M, Moreira L, Daros AC, Oliveira CS. Experimental investigation of postoperative use of medication in refractive surgery. Arq Bras Oftalmol 2005; 68: 223-227.
-
Chung JL, Seo KYS, Yong DE, Mah FS, Kim TI, Kim EK, Kim JK. Antibiotic susceptibility of conjunctival bacterial isolates from refractive surgery patients. Ophthalmology 2009; 116: 1067-1074.
-
Scheld WM. Maintaining fluoroquinolone class efficacy: review of influencing factors [editorial]. Emerg Infect Dis 2003; 9: 1-9.
-
Neu HC. Microbiologic aspects of fluoroquinolones [review]. Am J Ophthalmol 1991; 112(suppl): 15S-24S.
-
Mather R, Karenchak LM, Romanowski EG, Kowalski RP. Fourth generation fluoroquinolones: new weapons in the arsenal of ophthalmic antibiotics. Am J Ophthalmol 2002; 133: 463-466.
-
Hyon J-Y, Joo M-J, Hose S, Sinha D, Dick JD, O’Brien TP. Comparative efficacy of topical gatifloxacin with ciprofloxacin, amikacin, and clarithromycin in the treatment of experimental Mycobacterium chelonae keratitis. Arch Ophthalmol 2004; 122: 1166-1169.
-
Abshire R, Cockrum P, Crider J, Schlech B. Topical antibacterial therapy for mycobacterial keratitis: potential for surgical prophylaxis and treatment. Clin Ther 2004; 26: 191-196.
-
Saravolatz LD, Leggett J. Gatifloxacin, gemifloxacin, and moxifloxacin: the role of 3 newer fluoroquinolones. Clin Infect Dis 2003; 37: 1210-1215.
-
Moshirfar M, Mirzaian G, Feiz V, Kang PC. Fourth-generation fluoroquinolone-resistant bacterial keratitis after refractive surgery. J Cataract Refract Surg 2006; 32: 515-518.
-
Hadden OB, Patel D, Gray TB, Morris AT, Ring CP. Multifocal lamellar keratitis following laser in situ keratomileusis. J Cataract Refract Surg 2007; 33: 144-147.
-
Peng Q, Holzer MP, Kaufer PH, Apple DJ, Solomon KD. Interface fungal infection after laser in situ keratomileusis presenting as diffuse lamellar keratitis. A clinicopathological report. J Cataract Refract Surg 2002; 28: 1400-1408.
-
Arshinoff SA, Mills MD, Haber S. Pharmacotherapy of photorefractive keratectomy. J Cataract Refract Surg 1996; 22: 1037-1044.
-
John T, Velotta E. Nontuberculous (Atypical) Mycobacterial keratitis after LASIK. Current status and clinical implications. Cornea 2005; 24: 245-255.
-
Wallace RJ, Jr, Brown BA, Griffith DE. Nosocomial outbreaks/pseudo-outbreaks caused by nontuberculous mycobacteria. Annu Rev Microbiol 1998; 52: 453-490.
-
Daines BS, Vroman DT, Sandoval HP, Steed LL, Solomon KD. Rapid diagnosis and treatment of mycobacterial keratitis after laser in situ keratomileusis. J Cataract Refract Surg 2003; 29: 1014-1018.
-
Holmes G, Bond GB, Fader RC, Fulcher SF. A cluster of cases of Mycobacterium szulgai keratitis that occurred after laserassisted in situ keratomileusis. Clin Infect Dis 2002; 34: 1039-1046.
-
Biswell R. Cornea. In: Vaughan D, Asbury T, Riordan-Eva P, eds, General Ophthalmology, 15th ed. Stamford, CT, Appleton & Lange 1999; 119-141.
-
Ford JG, Huang AJW, Pflugfelder SC, Alfonso EC, Forster RK, Miller D. Nontuberculous mycobacterial keratitis in south Florida. Ophthalmology 1998; 105: 1652-1658.
-
Eiferman RA, Stagner JI. Intraocular penetration of amikacin; iris binding and bioavailability. Arch Ophthalmol 1982; 100: 1817-1819.
-
Alexandrakis G, Alfonso EC, Miller D. Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology 2000; 107: 1497-1502.
-
Hamam RN, Noureddin B, Salti HI, Haddad R, Khoury JM. Recalcitrant post-LASIK Mycobacterium chelonae keratitis eradicated after the use of fourth-generation fluoroquinolone. Ophthalmology 2006; 113: 950-954.
-
Lee SB, Oliver KM, Mohan SK, Slomovic AR. Fourth-generation fluoroquinolones in the treatment of mycobacterial infectious keratitis after laser-assisted in situ keratomileusis surgery. Can J Ophthalmol 2005; 40: 750-753.
-
Abshire R, Cockrum P, Crider J, Schlech B. Topical antibacterial therapy for mycobacterial keratitis: potential for surgical prophylaxis and treatment. Clin Ther 2004; 26: 191-196.
-
Hyon JY, Joo MJ, Hose S, Sinha D, Dick JD, O’Brien TP. Comparative efficacy of topical gatifloxacin with ciprofloxacin, amikacin, and clarithromycin in the treatment of experimental Mycobacterium chelonae keratitis. Arch Ophthalmol 2004; 122: 1166-1169.
-
Höfling-Lima AL, de Freitas D, Mello Sampaio JL, Leão SC, Contarini P. In Vitro activity of fluoroquinolones against Mycobacterium abscessus and Mycobacterium chelonae causing infectious keratitis after LASIK in Brazil. Cornea 2005; 24: 730-734.
-
Majid Moshirfar, MD, Jay J. Meyer, MPH, Ladan Espandar. Fourth-generation fluoroquinolone-resistant mycobacterial keratitis after laser in situ keratomileusis. J Cataract Refract Surg 2007; 33: 1978-1981.
-
de la Cruz J, Behlau I, Pineda R. Atypical mycobacteria keratitis after laser in situ keratomileusis unresponsive to fourth-generation fluoroquinolone therapy. J Cataract Refract Surg 2007; 33: 1318-1321.