UPDATE REVIEW
Ocular pharmacology during gestation and lactation (II)
GIL RUIZ MR1, ORTEGA USOBIAGA J2, GIL RUIZ MT3, CORTÉS VALDÉS C4
1 Ph.D.
in Medicine and Surgery. Deputy Ophthalmology Service. Nuestra Señora del Prado
Hospital. Talavera de la Reina (Toledo), Spain.
2 Ph.D. in Medicine and Surgery. Specialist in Ophthalmology. Baviera
Clinic – European Ophthalmological Institute, Bilbao (Bizkaia), Spain.
3 Ph.D. in Medicine and Surgery. Clinical Analysis Service Chief.
Nuestra Señora del Prado Hospital. Talavera de la Reina (Toledo), Spain.
4 Ph.D. in Medicine and Surgery. Ophthalmology Dept. Chief.
University General Hospital «Gregorio Marañón». Madrid, Spain.
INTRODUCTION
A large percentage of patients who visit the ophthalmology practice are within the fertile age range. For this reason, the physician must have in-depth knowledge of the impact that pregnancy can have on ocular physiology and previously existing diseases, but must also be fully aware of the levels of safety of each drug for gestation and breastfeeding. This is the only way to minimize the secondary effects that treatment of pregnant women can have on the developing fetus or the newborn.
By way of continuation of the first part of this article, this second part describes the levels of safety in gestation and breastfeeding for the drugs most commonly utilized in ophthalmology. The letters A, B, C, D and X in brackets indicate the safety category of the active principles according to the FDA.
1. ANTI-INFLAMMATORIES
Anti-inflammatories constitute a heterogeneous group of chemical substances divided in three main groups: steroid anti-inflammatories (corticoids), non-steroid anti-inflammatories (NSAID) and immunomodulators.
Such pharmacological abundance allows us to adapt a treatment to each patient –particularly during gestation– as we have a number of chemical structures available featuring different properties, indications and of course degrees of efficacy, tolerance and administration routes.
1.1. Corticoids
On the basis of bioavailability, place of action and intrinsic potency of the molecule, corticoids used in ophthalmology are classified in three groups: weak, such as medrisone (not available in Spain as an individual component), intermediate such as cortisone, hydrocortisone, fluorometholone and deflazacort, and potent such as prednisone, prednisolone, dexamethasone, triamcinolone, betamethasone and rimexolone (1). In addition, medroxyprogesterone is a progestogen with low anti-inflammatory potency but with anti-collagenase activity (table 1).
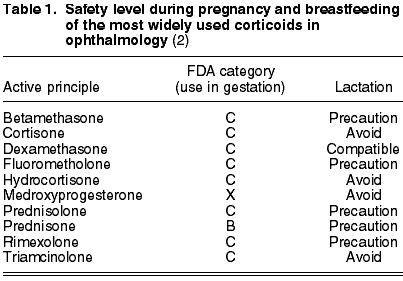
Prednisone has been classified by the FDA in category B, while the remainder of corticoids are in level C because animal studies have demonstrated adverse effects in the fetus although there are no adequate or properly controlled studies in pregnant women, or studies on animals have not been carried out and there are no adequate, well controlled studies on pregnant women. Said drugs must be administered only if the possible desired benefit justifies the potential risk for the fetus.
Dexamethasone is compatible with the lactation period. In contrast, triamcinolone, cortisone, hydrocortisone and deflazacort should be avoided. As regards the remainder of active principles, the physician must determine whether or not they are strictly necessary.
Medroxyprogesterone is totally contraindicated during pregnancy and breastfeeding and is a category X drug.
1.2. Non-steroid anti-inflammatory drugs
According to the FDA, NSAIDs should not be prescribed for pregnant women. Studies on animals have shown that, when the administered amounts are large enough to produce negative effects on the mother, the growth rate and weight of the fetus diminishes (3). The NSAIDs most widely utilized in Ophthalmology are: diclofenac (B), flurbiprofen (D), indomethacin (D), ketorolac (C), pranoprofen (B) and ibuprofen (B/D) (Table 2). The latter active principle should be avoided during the first and second quarter of pregnancy unless strictly necessary. On the contrary, it is completely contraindicated in the third quarter as it is related to cardiopulmonary toxicity and kidney dysfunction in the fetus. In addition, it could expose the mother and the fetus to extended hemorrhage times, anti-aggregating effects and inhibition of uterus contractions (2).
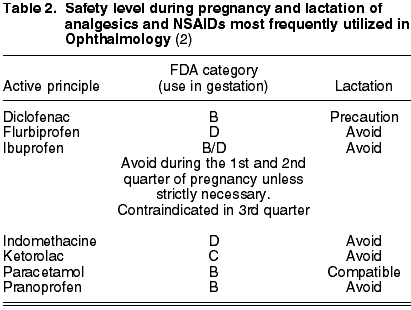
To conclude, NSAIDs should be administered only if the expected desired benefit justifies the dangers for the fetus. Whenever possible, it is preferable to substitute NSAIDs by paracetamol (B), a safe analgesic during gestation and compatible with the possibility of breastfeeding. Diclofenac can be utilized with caution during the lactation period, but the rest are forbidden.
1.3. Immunomodulators
As for immunomodulator drugs commonly utilized in Ophthalmology, there are four large groups: T-cell inhibitors (cyclosporine, tacrolimus), antimetabolites (metotrexate, azatioprin, mycophenolate mofetil), alkylating (cyclophosphamide, chlorambucyl) and biological therapies (Table 3). The latter are divided in anti-tumoral necrosis factor (adalimumab, etanercept, infliximab) and interleukin 2 inhibitors (daclizumab) (4).
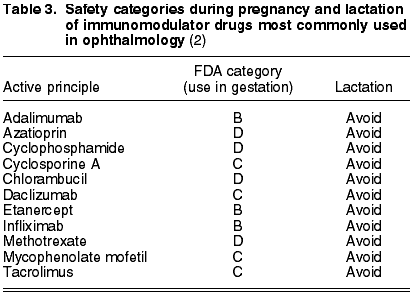
Cyclosporine A (C) is the most frequently prescribed immunomodulator drug in ophthalmology. It has not been studied whether it is excreted in maternal milk after topical administration, although it is known that when administered through other routes it is eliminated through the milk. Even though concentrations of cyclosporine A are not detected in blood after topical administration, it is not advisable to prescribe it during lactation (5).
Generally, immunomodulating drugs are not convenient during pregnancy as most have the D level of safety according to the FDA. Specifically, metotrexate exhibits a teratogenic and abortive risk and it is advised to utilize contraception methods up to month 6 after the last dose in women and month 3 in men.
Azathioprin can delay intrauterine growth and produce mutagenicity or teratogenicity, contraception being proposed in this case as well. Chlorambucyl develops infertility, increases the risk of leukemia and is also teratogenic.
Finally, in all biological therapies it is recommended to avoid pregnancy and lactation up to 6 months after ending the treatment (5).
2. ANTIALLERGICS AND VASOCONSTRICTORS
It is quite common for ophthalmologists to see patients with allergy symptoms. The ocular discomfort they describe and the difficulties they experience in their daily activities make it necessary to treat said symptoms independently of pregnancies.
Vasoconstrictor and antiallergic drugs are prescribed in the presence of allergic clinic. The former group includes phenylephrine (C), nafazolin (C), oximethazolin (B) and tetrizolin (C) (table 4).
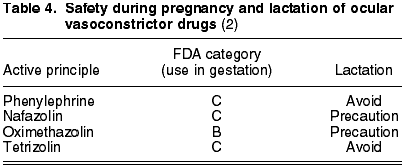
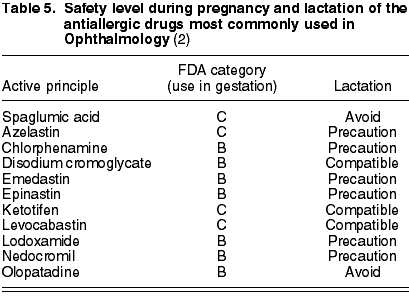
The antiallergic active principles are the following: histamine receptor antagonists (chlorphenamine (B), emedastin (B) and levocabastin (C)), that inhibit the degranulation of mastocytes [spaglumic acid (C), disodium cromoglycate (B), lodoxamide (B) and nedocromil (B)] and multiaction drugs [azelastin (C), epinastin (B), olopatadin (B) and ketotifen (C)] (6). Levocabastin, disodium cromoglycate and ketotifen are compatible with lactation. Spaglumic acid, olopatadin, phenylephrine and tetrizolin are forbidden. The remaining chemical agents should be used only after the ophthalmologist has assessed their benefits (table 5).
3. OCULAR HYPOTENSORS
There are no clinical trials on anti-glaucomatous drugs during pregnancy. In general it is preferable to suspend medical treatment temporarily and closely follow-up the patient provided that the progression risk is low (7).
Prostanoids (latanoprost, travoprost, bimatoprost) are forbidden. Teratogenic effects have been observed in animals and could stimulate uterine contractions (8). The recently marketed tafluprost does not have sufficient trials to classify its safety during pregnancy (table 6).
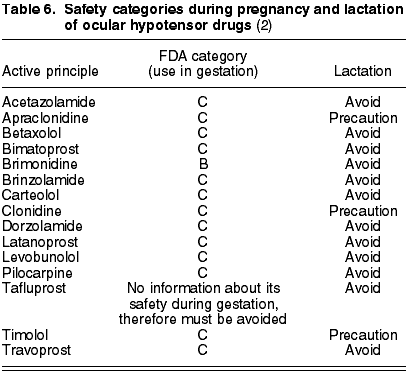
Brimonidine is the only ocular hypotensor with safety category B according to the FDA. The rest of ·2-adrenergic agonists (apraclonidine and clonidine) are in level C.
Cholinergic agonists, with pilocarpin as the only anti-glaucomatous example in the market, are in safety category C.
b
-blockers (timolole maleate, carteolol, levobunolol and betaxolol) are in level C. Even so and extrapolating the experience with these drugs via the systemic route, there are numerous studies that support their use, on the basis of their indication for treating arterial hypertension or arrhythmia during pregnancy. On the basis of this argument, the European Glaucoma Society proposed b-blockers as drugs of first choice for pregnant patients with high progression risk (7).Carbonic anhydrase inhibitors (acetazolamide, brinzolamide and dorzolamide) have also been classified by the FDA in category C (table 6).
However, glaucoma specialists recommend avoiding brimonidine during the latter months of pregnancy due to the possible risk of respiratory depression in the newborn, as well as b-blockers to reduce as much as possible the potential risks derived from the systemic effects of medication in the newborn. Although there is not enough clinical evidence on these two recommendations, the severity of their consequences call for prudency in their use and the search for alternatives whenever possible.
The American Pediatric Academy has approved the administration of b-blockers and carbonic anhydrase inhibitors for lactating mothers, although with caution. a2-adrenergic agonists must be avoided as they are contraindicated for the pediatric population (8).
4. ARTIFICIAL TEARS
The dynamic nature of the lachrymal tear and its complex composition, with multiple individual variations, render the reproduction of tears in laboratories practically impossible, making it necessary to develop tear substitutes. The main component of artificial tears is water with the addition of an active principle and a range of elements which stabilize the pH and regulate the tear stability (9). For this reason there are no adverse effects described after the use of humidifying tear treatment excepting those derived from allergy to any of the components or preservatives.
There is no references to complications arising from the prescription of lachrymal substitutes in pregnant and breastfeeding women. Even so, it is preferable to utilize the products which have the longest experience as there are no clinical trials for this period of life.
The most common active principles in ocular lubricants are: carbomere (C), carmelose (C), hypromelose (C), paraffin (C), lanolin (C), polyvinyl alcohol (C) and povidone (C). There is not enough experience with hyaluronic acid during gestation and therefore the risk/benefit ratio must be assessed (table 7).
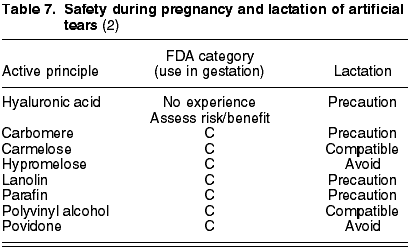
Carmelose and polyvinyl alcohol have proved to be compatible with breastfeeding. Hyaluronic acid, carbomere, paraffin and lanolin require caution in their use, while hypromelose and povidone should be avoided (table 7).
5. ANTIANGIOGENICS
Antiangiogenic drugs constitute a set of highly current active principles useful in ocular diseases associated to vascular endothelial growth factor such as choroidal and retinal neovascularization, macular edema, intraocular inflammatory process and corneal and iridian neovascularization (10). Some antiangiogenics are approved by the FDA for non-intraocular uses such as intravitreal bevacizumab or triamcinolone. Due to the fact that these drugs are used as a compassionate medication, there are no studies accepted by international bodies supporting their safety and efficacy, even less in gestating women (11).
While triamcinolone is in category C, bevacizumab is contraindicated. For the latter there are no data about pregnant women and animal studies have exhibited reproductive toxicity, including malformations (table 8). It is known that immunoglobulins G (IgG) traverse the placenta and are assumed to inhibit angiogenesis in the fetus; therefore IgG are expected to cause severe birth defects if administered during pregnancy (2).
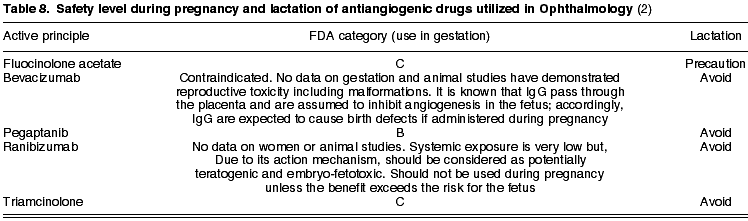
In contrast, ranibizumab and pegaptanib have been approved by the FDA for treating age-related macular degeneration of the exudative type, although they have not been studied in pregnant women. Pegaptanib is in Category C. As for ranibizumab, although its systemic concentrations are low, it should be considered as potentially teratogenic and embryo-fetotoxic due to its action mechanism (2). There are no data about women or studies on animals. It should not be used in pregnancy unless the benefit exceeds the fetal risk (table 8).
Fluocinolone acetate (C) was accepted by the FDA in 2005 as an intravitreal device for treating macular edema associated to chronic uveitis. It is eliminated through maternal milk and therefore, as with the remainder of antiangiogenics, its use during lactation is not allowed.
6. VITREOUS SUBSTITUTES
Vitreous substitutes are frequently utilized in vitreoretinal surgery to maintain the ocular anatomy. At present there are perfluorocarbonated liquids (perfluoro-n-octane, perfluoromethyldecalin, perfluorotributylamine, perfluorofenantrene), non-expandable intraocular gases (air, xenon), expandable gases (sulphur hexafluoride, perfluoromethane, perfluoroethane, perfluoropropane) and intraocular silicone (polydimethylsilicone, fluorosilicone and heavy silicones) (12). There is no record of clinical data about pregnant or lactating women and accordingly these substitutes should be avoided in both cases, or at least should be utilized with caution (table 9).

7. BIOADHESIVES
Currently available bioadhesives are cyanoacrylates, acrylic polymers and biological adhesives. The latter are made up of fibrinogen, thrombin and calcium (13). As in the previous pharmacological group, there is no experience during pregnancy or lactation (table 10).

8. BOTULIN TOXIN
The active principle is an exotoxin synthesized by Clostridium botulinum, a grampositive sporulated anaerobic bacteria. This toxin is able to develop a reversible flaccid muscular paralysis (14).
There are 2 serotypes; serotype A exhibits a safety level of C whereas serotype B involves unknown potential risks for humans, making its use unadvisable for pregnant patients unless strictly necessary (2). Both serotypes are macromolecules which, under normal conditions, would not pass through the blood-brain barrier or the placenta but, due to the absence of experience in pregnant and lactating women, its use is not advisable (table 11).

9. VISCOELASTICS
Viscoelastics are necessary in multiple ophthalmological surgical procedures and contain one or more of the following substances in various concentrations: sodium hyaluronate, chondroitin sulphate and hydroxypropylmethylcellulose (14). At this time there is no research on the use of viscoelastics in pregnant or lactating women and therefore its utilization is not recommended (table 12).

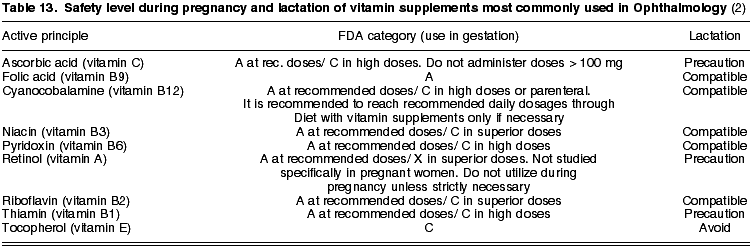
10. VITAMIN SUPPLEMENTS
At present there are a number of vitamin complexes in the market which, as a supplement to our food intake, constitute a balanced supply of nutrients and antioxidants. Supplements also increase the defense mechanisms of the body against oxidative processes which are a potential hazard for the visual function, as is the case of ultraviolet radiation.
Vitamin complexes comprise carotenoids (lutein, zeaxanthin, astaxanthin), vitamins A (retinol), C (ascorbic acid), E (d-a-tocopherol) and group B (B1 or thiamin, B2 or riboflavin, B3 or niacin, B6 or pyridoxine, B9 or folic acid and B12 or cyanocobalamin); minerals (zinc, manganese, selenium, copper) and Omega-3 essential fatty acids such as DHA or docosahexaenoic acid and EPA or eicosapentaenoic acid).
Vitamin supplements have multiple functions, as many as components included in their formulation. Accordingly, carotenoids are pigments located in the macula with antioxidant properties and which also protect photoreceptors. Vitamins A, C and E normalize numerous metabolic reactions, delaying the ageing process activated by free radicals. Vitamin group B is necessary for the proper function of the nervous system and eyes because it provides the body the energy it needs for carrying out adequately various metabolic reactions. In turn, minerals are part of the cell antioxidant system, and finally fatty acids are essential for the retina (2).
With the exception of tocopherol, which exhibits a C fixed safety level, the rest of vitamins have different safety ratings according to the dosage being administered. Specifically, vitamin A can reach category X when exceeding the recommended dosage (table 13). For this reason and considering the absence of sufficient studies on pregnant patients, it is advised to obtain said active principles through healthy and well-balanced food intake during pregnancy and lactation, administering vitamin supplements only in extreme cases (2).
REFERENCES
-
Gil MR, Cortés C. Farmacología de la Inflamación y Analgesia en Oftalmología. En: Cortés C, Arias A, Encinas JL, García J. LXXXIII Ponencia Oficial de la Sociedad Española de Oftalmología. Farmacología Ocular. Madrid: Industria Gráfica MAE SL; 2007: 163-177.
-
Vademecum Internacional. 50ª edición; Madrid: CMP Medicom; 2009.
-
Ministerio de Sanidad y Consumo. Información de medicamentos. Consejos al paciente. 11ª edición; Madrid: Einsa; 1994.
-
Díaz D, Benítez del Castillo JM, Arriola P. Corticoides sistémicos e inmunoterapia en oftalmología. En: García-Sánchez J, García-Feijoó J. Oftalmomecum 2009. Barcelona: Publicaciones Permanyer; 2009: 111-127.
-
Arriola P, Díaz D, Benítez del Castillo JM. Antiinflamatorios. Corticosteroides, antiinflamatorios no esteroideos, otros agentes moduladores de la inflamación. En: García-Sánchez J, García-Feijoó J. Oftalmomecum 2009. Barcelona: Publicaciones Permanyer; 2009: 51-63.
-
Gil MR, Cortés C. Antialérgicos y vasoconstrictores. En: García-Sánchez J, García-Feijoó J. Oftalmomecum 2009. Barcelona: Publicaciones Permanyer; 2009: 65-70.
-
European Glaucoma Society. Terminología y pautas para el glaucoma. 2.ª edición; Savona: Dogma; 2003.
-
Urcelay JL. Tratamiento médico antiglaucomatoso en mujeres gestantes y lactantes. En: Cortés C, Arias A, Encinas JL, García J. LXXXIII Ponencia Oficial de la Sociedad Española de Oftalmología. Farmacología Ocular. Madrid: Industria Gráfica MAE SL; 2007: 297-299.
-
Benítez del Castillo JM, Díaz D, Arriola P. Lágrimas artificiales. En: García-Sánchez J, García-Feijoó J. Oftalmomecum 2009. Barcelona: Publicaciones Permanyer; 2009: 81-87.
-
Bañuelos J, García MC, Gili P, Arias A. Antiangiogénicos. En: Cortés C, Arias A, Encinas JL, García J. LXXXIII Ponencia Oficial de la Sociedad Española de Oftalmología. Farmacología Ocular. Madrid: Industria Gráfica MAE SL; 2007: 301-324.
-
Bañuelos J, Gili P, Arias A. Antiangiogénicos. En: García-Sánchez J, García-Feijoó J. Oftalmomecum 2009. Barcelona: Publicaciones Permanyer; 2009: 89-96.
-
Encinas JL, Bañuelos J. Sustitutivos vítreos. En: Cortés C, Arias A, Encinas JL, García J. LXXXIII Ponencia Oficial de la Sociedad Española de Oftalmología. Farmacología Ocular. Madrid: Industria Gráfica MAE SL; 2007: 405-443.
-
Cortés C, Gil MR, Cortés I. Adhesivos en Oftalmología. En: Cortés C, Arias A, Encinas JL, García J. LXXXIII Ponencia Oficial de la Sociedad Española de Oftalmología. Farmacología Ocular. Madrid: Industria Gráfica MAE SL; 2007: 445-449.
-
Encinas JL, Gómez de Liaño R, Muñoz AM. Sustitutivos vítreos. Bioadhesivos. Toxina botulínica. Viscoelásticos. En: García-Sánchez J, García-Feijoó J. Oftalmomecum 2009. Barcelona: Publicaciones Permanyer; 2009: 97-110.