REQUESTED COMMUNICATION
Chronic canaliculitis, a series of 18 cases
SEVILLANO TORRADO C1, MURUZABAL ZALDIBAR N2, FERNÁNDEZ HERMIDA R3
Ophthalmology Service. Cruces Hospital,
Barakaldo, Spain.
1 Graduate in Medicine. Ophthalmology Service, Pontevedra Hospital
Complex.
2 Graduate in Medicine. Ophthalmology Service. Cruces Hospital,
Barakaldo, Spain.
3 Ph.D. in Medicine. Ophthalmology Service, Cruces Hospital,
Barakaldo. Orbit and Oculoplasty Unit.
RESUMEN
Introduction: The purpose of this paper is to collect information on 16 cases of chronic canaliculitis to study epidemiology, presentation and most frequent etiology, as well as compare this information with published literature and discuss the most appropriate treatment.
Results: Our series is comparable to the rest of published series, finding Actinomyces as the most frequent germ and canaliculotomy as the most effective treatment. In addition, this paper describes other germs which cause said frequently under-diagnosed pathology.
Key words: Chronic canaliculitis, Actinomyces, treatment.
INTRODUCTION
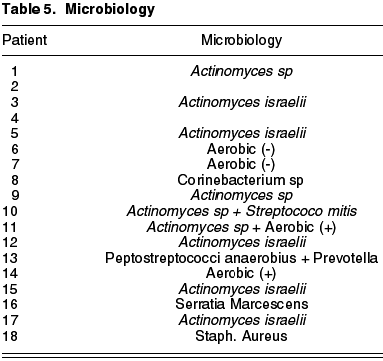
Chronic canaliculitis is an infrequent although highly under-diagnosed disease characterized by chronic purulent secretion and tearing (1-9,11,12). The germ which has been named in most cases is Actinomyces (1-9,12), a gram-positive anaerobic bacillus, although optionally aerobic, present in the flora of mucous membranes (7,11) (fig. 2). The following germs in order of frequency are Nocardia and staphylococci, according to various authors (1,2,6).
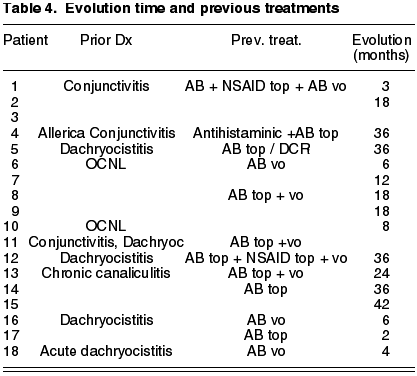
In addition, as the symptoms are usually subclinic, the disease is confused with other diagnostics (Table 4) and the mean delay in discovering actinomicosis is estimated to be about 21 months (11).
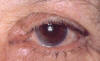
Fig. 1: End dilatation, purulent secretion and Tumoration, in this case
bicanalicular.
The predisposing factors for suffering said disease comprise a range of palpebral pathologies such as blepharitis or malpositions (2), female sex and immunodepression (11). The female sex is clearly related and it has been postulated that this is due to two main factors: smaller pathway diameter and hormonal factors (1) which contribute to alteration of the canaliculum epithelium (diverticles, scaling, etc.). An author (1) has not found any predisposing factor.
The clinics, which are subdued, are mainly characterized by epiphora and purulent secretion (1-9) (Tables 2 and 3) (fig. 2). A characteristic is the permeable lachrymal pathway (nearly 100%) despite exhibiting dachryolites. These are non-pathognomic for Actinomyces yellow sulphur granules (fig. 2), which appear in >90% of all chronic canaliculitis (2,5).
The diagnostic is mainly clinical, although it can be supported by microbiological culture which will give us an etiological diagnostic and by high-frequency ultrasound echography which evidences the dachryolites and excludes other pathologies such as tumors (10).
The differential diagnostic (1-9,12) must be made with canalicular obstructions arising from cicatricial conjunctivitis i.e., Ocular Penfigoid Cicatricial, VHS or VHZ infections, trachoma, also with styes or medial Meibomian cysts which can cause mechanical obstructions. To differentiate these from acute or chronic dachryocistitis or mucocele, in addition to the location we must bear in mind that the pathway is frequently obstructed.
Despite being rare, canalicular tumors (which account for 0.5% of orbit and para-orbit tumors), usually exhibit differences such as bloody secretion, induration towards the nasal area, globe displacement and exhibit alterations in imaging tests [20 mHz echography, TC, dachryocistography (12), etc.].
The treatment will be discussed further down although the main lines are:
-
Oral antibiotic therapy: oral penicillin 2g per day for 3-6 months, following guidelines for systemic actinomicosis (frequently recurring) (10).
-
Irrigation with antibiotics (6).
-
Curettage (4).
-
Canaliculotomy (1,2,5,12).
with combinations between these. We propose canaliculotomy with or without curettage as the best option.
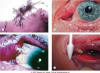
Fig. 2: A. Israelii gram stain (a); edema, secretion and tumoration of both
canaliculi (b); end dilatation and mucopurulent discharge (c); dachryolites
obtained by pressing the canaliculum (d); With authorization of Elsevier 2007
(in Clinical Ophthalmology, 6th edition; J. Kanski; page 160).
DESIGN AND METHODS
All the chronic canaliculitis cases reaching the Cruces Hospital (Barakaldo) and the Ophthalmology Surgical Clinical Institute (Bilbao) between February 1998 and December 2008, and the Pontevedra Hospital Complex between October 2007 and November 2009 were collected retrospectively, checking whenever possible to determine the existence of relapses.
Canaliculotomies were performed in the surgery after injecting anesthetic and aided by a 0/0 intubation probe as a tutor. The samples were sent to Microbiology for aerobic, anaerobic culture and Giemsa staining. The pathway was not reconstructed after surgery.
RESULTS
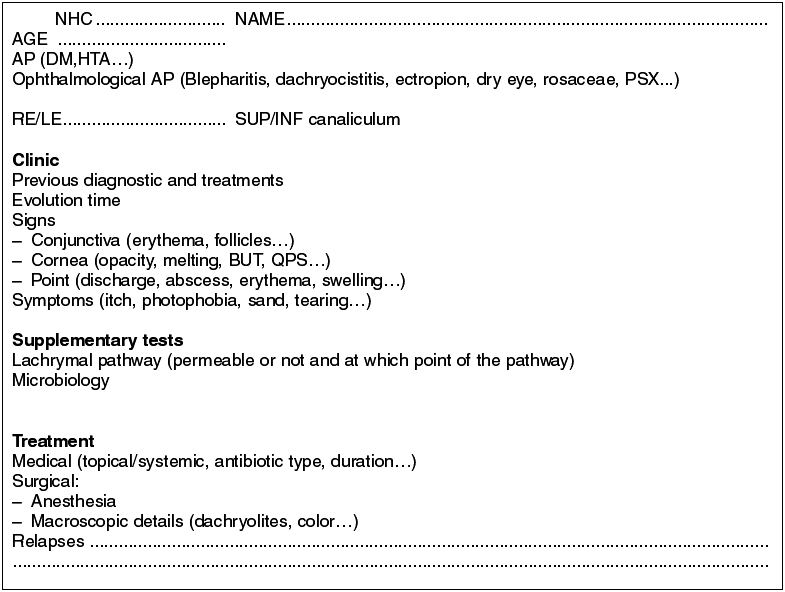
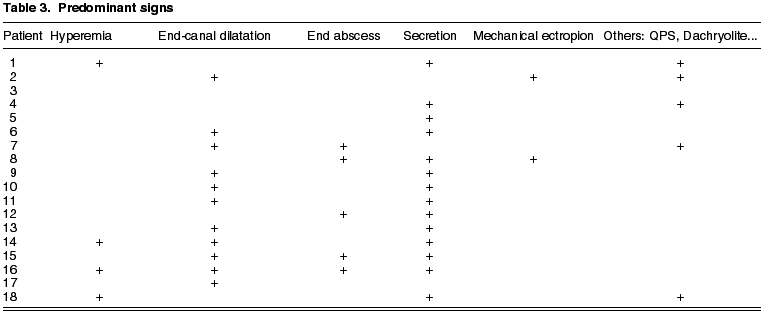
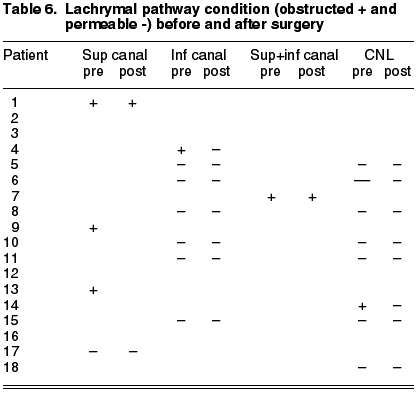
This is a series of cases of 18 patients having epidemiologies matching those described in previous publications. The mean age is of 59.2 years (range 19-88), prevalence in women (5:1), 68% in Inferior canaliculum, unilateral condition and frequently underdiagnosed (mean duration of symptoms 21.43 months, range 2-36) (Table 3). The most frequently recorded symptom was epiphora (55%); and end dilatation signs (61%) and chronic purulent secretion (77.7%) (fig. 1) (Tables 1 and 2). 50% of the cases had received topical antibiotic therapy which proved ineffective. One patient was diagnosed with chronic canaliculitis but oral antibiotic therapy was not effective either (Table 3).
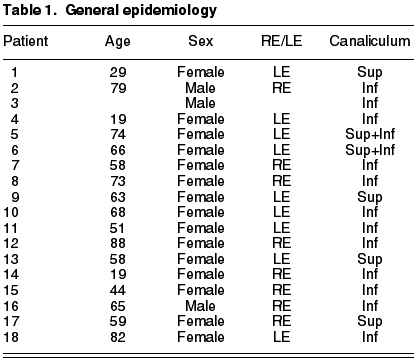
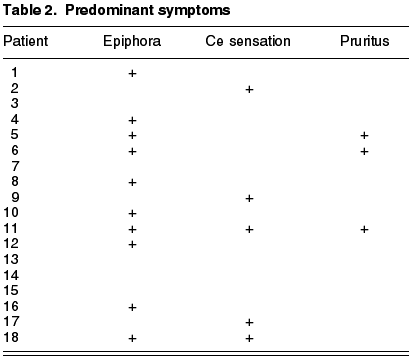
In what concerns microbiology, Actinomyces was isolated in 9 patients (50%), finding other pathogens such as Peptostreptococci aerobius, Streptococci mitis, Prevotella, Serratia marcenscens or Corynebacterium sp. Two of the samples were cultured only in aerobic ambient with negative result, and perhaps in anaerobic medium the actinomyces would have prospered. The percentage of dachryolites or sulphur granules was of 100%.
The lachrymal pathway was obstructed in four patients (22.2%), and after surgery only two remained unchanged. In our series there were no iatrogenic stenosis or any type of relapse, and only the aforementioned two patients complained of epiphora. The mean follow-up for all the series was of 28.21 months (range 6 months-9 years).
DISCUSSION AND CONCLUSIONS
This paper presents a series of 16 cases of chronic canaliculitis which, after the 40-case series by Vecsei (1), is the longest one published in the literature. The epidemiological data match those of other publications.
Only the Vecsei series (1) found a majority of superior canaliculi, whereas the rest (2-6,12) found like us a majority of inferior canaliculi. Although initially there are no predisposing local factors (1), Anand (2) found palpebral pathology in about 40%. We found palpebral pathology in 4 subjects (25%; 2 with anterior staphylococci blepharitis and a further 2 with lower eyelid malpositions). In our series, said entity continues to be an underdiagnosed disease (1,3,5,12), with a mean evolution of nearly two years [greater than the literature, only Briscoe (5) refers a mean of 3 years].
In what concerns microbiology, cultures revealed 50% of Actinomyces, a percentage which exceeds other series [Anand (2) found 14%, Mohan (6) 17%, Briscoe (5) 30% and Vecsei (1) 23%] even though Actinomyces is recognized as a difficult germ to culture in a lab (2,3,5,12). It is important to consider Actionmyces for the microbiological cultures and to use anaerobic supports. In addition, we observed that the presence of dachryolites is not exclusive to Actinomyces as in our experience they appeared in 100%, the same as Briscoe’s finding (5) [against 33% in the Anand series (2)]. We did not evidence Nocardia and only one staphylococci, the second most frequent germs according to the authors (1,2,6), but we did find other pathogens as yet undescribed, such as Serratia marcenscens, Peptostreptococci aerobius, Streptococci mitis, Prevotella or Corynebacterium sp.
As regards treatment, there are several available options. Mohan (6) found healing without relapse for 12 months irrigating the canaliculum with reinforced antibiotics (although only 2 cases were Actinomyces), while Pavilack (4) preferred curettage without canaliculotomy, obtaining good results but requiring 2 interventions in half of the cases. Vecsei (1) stated that conservative treatment worked in only 10% of patients.
Several published cases report that, after several irrigations with antibiotic therapy, it was necessary to perform curettage or posterior surgery (2,9,12). In our series, all the patients underwent canaliculotomy and none experienced relapses or complications during the follow-up period. As with the series of Briscoe (5) and Demant (12), it was not necessary to reconstruct the pathway after surgery and we did not observe iatrogenic stenosis. Anand (2) reported 2 cases of this type but specified that these patients had previously received up to 4 lachrymal sac cleanses, indicating that the risk of epiphora is not increased due to post-surgical causes. Vecsei (1) found 20% of patients with epiphora after the intervention (albeit in the permeable pathway), while we did not found new stenosis, with two patients who already exhibited it and a further two in which surgery was enough to reconduct the pathway.
It must be pointed out that the limitations of this study include the small number of cases (18) due to the low frequency of this pathology. In addition, the limitations derived from the retrospective nature thereof, such as the lack of some epidemiological data. However, a positive aspect of the study is that it is the second largest published series, although there is little supporting literature as demonstrated by the short bibliography.
REFERENCES
-
Vécsei VP, Huber-Spitzy V, Arocker-Mettinger E, Steinkogler FJ. Canaliculitis: difficulties in diagnosis, differential diagnosis and comparison between conservative and surgical treatment. Orbit. 2004 Mar; 23(1): 19-26.
-
Anand S, Hollingworth K, Kumar V, Sandramouli S. Canaliculitis: the incidence of long-term epiphora following canaliculotomy. Ophthalmologica. 1994; 208(6): 314-7.
-
Varma D, Chang B, Musaad S. A case series on chronic canaliculitis. Orbit. 2005 Mar; 24(1): 11-4.
-
Pavilack MA, Frueh BR. Through curettage in the treatment of chronic canaliculitis. Arch Ophthalmol. 1992 Feb; 110(2): 200-2.
-
Briscoe D, Edelstein E, Zacharopoulos I. Actinomyces canaliculitis: diagnosis of a masquerading disease. Graefes Arch Clin Exp Ophthalmol. 2004 Aug; 242(8): 682-6.
-
Mohan ER, Kabra S, Udhay P, Madhavan HN. Intracanalicular antibiotics may obviate the need for surgical management of chronic suppurative canaliculitis. Indian J Ophthalmol. 2008 Jul-Aug; 56(4): 338-40.
-
Singh CN, Thakker M, Sires BS. Pyogenic granuloma associated with chronic Actinomyces canaliculitis. Ophthal Plast Reconstr Surg. 2006 May-Jun; 22(3): 224-5.
-
Melero P, Alvarez M, Llanos A, Pérez JM, Salaverri F, Cisterna R. Canaliculitis caused by Actinomyces israelii. Enferm Infecc Microbiol Clin. 1994 Feb; 12(2): 109-10.
-
Milagro A, Moles B, Ferrer I, Cruz Villuendas M, Marcuello B, Fernández J. Chronic unilateral canaliculitis Enferm Infecc Microbiol Clin. 2000 Jan; 18(1): 41-2.
-
Tost F, Bruder R, Clemens S. Clinical diagnosis of chronic canaliculitis by 20-MHz ultrasound. Ophthalmologica. 2000; 214(6): 433-6.
-
Harrison: principios de medicina interna. Ed Mc GrawHill, 16ª edición, 2005. Vol I, pág. 1044-47.
-
Demant E, Hurwitz JJ. Canaliculitis: review of 12 cases. Can J Ophthalmol. 1980 Apr; 15(2): 73-5.