UPDATE REVIEW
Neovascular glaucoma
RUIZ-CASAS D1, CABARGA-NOZAL C2, MUÑOZ-NEGRETE FJ3
1
Graduate in Medicine and Surgery. MIR 4th year of Ophthalmology. Ramón and Cajal
Hospital, Madrid.
2 Ph.D. in Medicine and Surgery. Attached to the Glaucoma Unit.
Ophthalmology Service. Ramón and Cajal Hospital, Madrid.
3 Ph.D. in Medicine and Surgery. Ophthalmology Service Chief, Ramón
and Cajal Hospital, Madrid. Ophthalmology Chair at Alcalá de Henares University,
Madrid.
CONCEPT
Neovascular glaucoma is an infrequent type of glaucoma (3.9%) which is a challenge for ophthalmologists because its difficult to manage and leads to considerable visual loss (1,2). To prevent neovascular glaucoma we must be aware of the diseases that could potentially cause it and perform adequate follow-up allowing for early diagnostic and treatment.
The term ‹neovascular glaucoma› was proposed by Weiss in 1963, although it has been given several names such as rubeotic, congestive and hemorrhagic glaucoma (3). It is a secondary glaucoma caused by the growth of a fibrovascular membrane at the level of the chamber angle which appears as a consequence of the angiogenic stimuli generated by ocular ischemia pathologies and (only in 3% of cases) by non-ischemic pathologies, generally due to inflammatory diseases (2).
Initially it appears as an apparently open angle glaucoma blocked by a thin fibrovascular membrane which finally retracts forming peripheral anterior synechiae which close the angle, transforming the condition to closed angle glaucoma (3).
PHYSIOPATHOLOGY
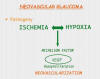
Tissue hypoxia secondary to retinal or choroidal ischemic pathology gives rise to an angiogenic stimuli involving the release of mainly the vascular endothelial growth factor (VEGF) although other related factors are also involved, including IGF-1 and 2, FGF, PDGF, IL-6.
VEGF is synthesized in at least 6 types of retinal cells, mainly in Müller cells. These factors promote a proliferation of abnormal neovessels without adventitious and a vascular endothelium without inter-cellular unions, facilitating the migration of pre-existing vessels and with increased vascular permeability (2,4-8).
Angiogenesis requires feasible capillaries for its development. For this reason, in pathologies with an extensive degree of retinal ischemia, the first location where neovessels appear is at the iris level. Said vessels, localized in a mesh of connective tissue with fibroblasts and miofibroblasts, grow behind the iris to subsequently advance towards the pupilar edge over the surface of the iris and finally in the chamber angle. Here, these vessels first cover it with a thin fibrovascular membrane over the trabecular mesh, which retracts and causes its closure due to irido-corneal apposition, much like a zip, causing angular closure which prevents the filtration of the aqueous humor and consequently increasing intra-ocular pressure (IOP) and secondary glaucomatous optic neuropathy (2,3,9).
Optic nerve damage is increased with higher IOP and ischemia due to the baseline pathology. Accordingly, in neovascular glaucoma (NVG) glaucomatous optic neuropathy progresses quickly (3,9) (fig. 1).
ETIOLOGY
Table 1 summarizes the diseases the can cause NVG.
The most common diseases causing neovascular glaucoma are described below.
1. Occlusive venous retinal pathology
NVG can appear in the context of ischemic central retinal vein occlusion (CRVO), ischemic Hemi-CRVO, multiple retinal venous branch occlusion (RVBO) (which affects large areas of the retina), or RVBO associated to another retinal ischemic pathology.
CRVO can be ischemic or non-ischemic. Only the ischemic variety can produce NVG by itself. The Central Vein Occlusion Study (CVOS) differentiates between ischemic CRVO (over 10 disc diameters (DD) of retinal capillary ischemia in angiofluoresceingraph (AGF)), non-ischemic (<10 DD) and undefined (due to extensive hemorrhage).
Of the ischemic or undefined CRVO, 35% developed neovessels in the iris (INV) or the angle (NVA) while only 10% of the non-ischemic CRVO developed it. The appearance of neovessels generally occurred within 3-5 months after the CRVO (3). The study demonstrated other risk factors for developing INV/NVA (visual acuity <20/200, retinal capillary ischemia > 30 DD or severe venous tortuosity). In addition, 34% of patients initially classified as non-ischemic CRVO progressed to the ischemic form within 3 years, and 48% of patients who developed INV/NVA of at least two hours extension were classified as non-ischemic CRVO. In addition, the study found that panphotocoagulation (PFC) did not diminish the risk of INV/NVA although it caused them to regress once present in 90% of cases. Accordingly, it recommended to perform PFC in the presence of INV/NVA covering over 2 clock hours (3,10-13).
Hayreh classified ischemic or non-ischemic CRVO utilizing anatomical and functional parameters, and likewise concludes that only 20% of CRVO are ischemic, of which 45% will develop NVG with the highest risk at 7-8 months (9).
In Hemi-CRVO only the ischemic forms are at risk of developing NVG and about 3% of these develop it.
In RVBO, the development of NVG is highly improbable as it requires a large angiogenic stimuli. Therefore RVBO have a very low risk of developing NVG unless they are associated to another ocular ischemic pathology. The Branch Vein Occlusion Study (BVOS) (14) defines ischemic ORVCR (> 5 DD of retinal capillary ischemia) and non-ischemic ORVCR (<5 DD), with the ischemic form being associated to a higher neovascularization risk (11-12,14).
2. Occlusive retinal arterial pathology
The risk of developing NVG in the retinal central artery occlusion (RCAO) is considerably lower than in CRVO, as the necrotic ischemic retina secondary to RCAO is unable to synthesize angiogenic factors (the ischemic retina can but not the necrotic retina).
Typically, it has been considered that RCAO develops INV in 20% of cases in a 4-5 week period as of the obstructive episode, with PFC being effective to achieve the regression of neovessels in 65% of cases (15). Hayreh referred that in RCAO ciliary-retinal collaterals can develop at the optic nerve level in 32% of cases, which can be confused with neovessels (9,15).
Eyes with baseline ischemic pathology exhibit a risk of developing neovascularization and NVG. This causes an increase of IOP in one eye with diminished perfusion pressure, and this involves increased risk of RCAO, choroidal infarcts and AION (anterior ischemic optic neuropathy). For this reason, occasionally RCAO can be a consequence instead of a cause of NVG (9,15).
Arterial branch occlusions have a minimum risk of developing neovessels, although these have been described, particularly in cases associated to diabetes mellitus (DM) (15).
3. Diabetic retinopathy (DR)
NVG is an expression of advanced diabetic retinopathy (DR) and can occur without retinal neovascularization, although it is more frequently associated to proliferative diabetic retinopathy (PDR). The prevalence of NVG in DR is 2%, but it increases up to 21% in PDR, in which the presence of INV can be of up to 65% (3,16).
Panphotocoagulation (PFC) is indicated in eyes with severe PDR (with disc neovessels >1/3 of disc diameter or vitreous/pre-retinal hemorrhage). It is also indicated in moderate or slight DRB or severe non-proliferative DR (NPDR), particularly in the aged. In these cases, PFC improves the visual prognosis (16).
4. Ischemic ocular syndrome (IOS)
This syndrome arises due to poor blood flow to the ocular globe caused by vessel obstruction which could arise at the level of the aorta, the carotid, the ophthalmic, ciliary or central retinal artery (CRA), with severe vessel obstructions being required.
IOS is generally diagnosed with carotid Eco-Doppler, but this test only assessed blood flow through the neck, which means that if the results are not conclusive and the diagnostic suspicion is high, vascular stenosis at other levels must be discarded with magnetic angio-resonance (angio-RM) o angio-CAT scan, reserving carotid angiography as the last diagnostic test (9,17).
Fluorescein angiography (FAG) of these patients rarely exhibits retinal capillary hypoperfusion, whereas choroidal vascular insufficiency is common. Accordingly, choroidal ischemia seems to be a powerful angiogenic stimuli on a par with retinal ischemia (9).
INV appear in 66% of patients (3). Even so, IOP can remain low due to the ischemia of the ciliary body which causes diminished production of aqueous humor. FAG may reveal signs of retinal capillary ischemia. In the cases with retinal ischemia photocoagulation can be utilized but when there only is uveal ischemia there are no scientific grounds for using it (10). PFC only produces a regression of INV in 36% of cases (18).
Revascularization surgery produces improvements in visual acuity (VA) and ophthalmoscopic signs in patients with IOS, but in advanced INV or NVG cases no VA improvements have been found (12,18) (table 2).
CLINIC AND CHRONOLOGY OF NEOVASCULAR GLAUCOMA
During the clinical course of the disease four main stages can be differentiated (3). These are:
1. Initial iris rubeosis stage
It exhibits neovessels in pupil area and/or in angle with normal IOP (although it could be high if associated to open angle primary glaucoma). Patients could remain asymptomatic during this stage.
2. Open angle stage
At this stage moderate iris and angle rubeosis can be observed, with non-visible fibrovascular tissue growth over the trabeculum which diminishes filtration and causes progressive IOP increase (although oscillations can appear). Also, inflammatory signs in the anterior chamber can be observed, coexisting with hyphema which exacerbates the process.
3. Closed angle stage
This stage is characterized by severe neovascularization in iris and angle with intense rubeosis. The contraction of the fibrovascular membrane causes the progressive closure of the angle. In this stage we may detect the presence of uveal ectropion, anterior peripheral synechiae, flat iris without crypts or valleys and abundant and thin mesh-shaped vessels. The stage courses with important anterior chamber inflammation, conjunctival and ciliar injection and corneal edema.
4. Final stage of congestive glaucoma
The angular closure is complete with very high IOP > 50 mmHg. In this stage patients refer from photophobia and diminished visual acuity up to intense pain with headaches, nausea and vomiting (fig. 2).
DIAGNOSTIC
The diagnostic should be as early as possible. However, it is generally arrived at after an episode of sudden ocular hypertension secondary to hyphema. The patient usually refers pain and upon exploration we find diminished VA, corneal edema, inflammation in anterior chamber and very high IOP.
1. Usual exploration
Anamnesis should be performed to determined the underlying pathology of the patient and request complete analytics to search for cardiovascular risk factors.
Determination of corrected VA.
Intrinsic Ocular Motility (IOM): relative afferent pupil defect (RAPD) may exist due to retinal ischemia.
Anterior poly biomicroscopy:
-
Iridian neovessels: particularly at the level of the pupil edge, in advanced states uveal ectropion may be present
-
Tyndall and anterior chamber turbidity, also hyphema may express.
Tonometry (IOP): It increases with closure of the angle but in ocular ischemia syndrome cases (IOS) it could be normal or low due to ciliary body ischemia
Gonioscopy: it is very important to asses angle amplitude and presence of neovessels (generally INV precede NVA, but the angle could be the first place where neovessels appear).
Funduscopy: to assess the baseline chorioretinal pathology as well as the optic nerve anatomy and retinal nervous fiber layer.
FAG: to diagnose the underlying pathology and to direct ablation treatment with PFC over the ischemic retina.
2. Special techniques
Anterior segment FAG: To diagnose INV before they become clinically evident.
Gonio-fluoresceingraph and Irido-fluoresceingraph: for early diagnosis of iridian and angle neovessels.
IGF (Indocyanine green angiography): Diagnostic assessment of choroidal ischemia.
ERG (Electroretinography): overall evaluation of the retina functional capacity and retinal ischemia degree.
Carotid ECO-DOPPLER: if no retinal pathology is found and IOS is suspected. In addition, Angio-RM and even carotid angiography can be resorted to if the suspicion is high and there is no vascular pathology at the neck level.
ECO-mode B: if the ocular fundus cannot be seen to discard intraocular tumors or retina detachment.
DIFFERENTIAL DIAGNOSTIC
Depending on the NGV stage:
1. In the initial stages the following diseases must be considered:
Inflammatory glaucoma: it also presents anterior chamber inflammation with Tyndall and turbidity, also dilated vessels can be observed but over a normal iris. No angular neovessels.
Fuchs heterochromic iridocyclitis: it can exhibit thin angular vessels, very different to those observed in NVG and without developing angular closure. These vessels can bleed after anterior chamber decompression or when performing gonioscopy.
Clear Iris: normal iridian vessels can be observed with radial trajectory in stroma.
Carotid-cavernous fistula: blood appears in Schlemm’s canal in addition to dilated episcleral vessels, pulsating exophthalmos and presence of audible breath.
2. In late NVG stages we must make a differential diagnostic
Closed angle glaucoma secondary to inflammation with peripheral anterior synechiae, traumatism or corneal irido-endothelial syndromes.
Primary closed angle glaucoma.
Highly evolved Phakogenic glaucoma.
Pseudoexfoliative glaucoma: it exhibits very high IOP and exceptionally neovessels could be associated.
TREATMENT
The treatment of this pathology should address both the baseline disease which causes the angiogenic stimuli and the IOP increase produced by angle neovascularization.
1. Etiological treatment
Angiogenic stimuli treatment:
VEGF is synthesized by the ischemic retina and therefore, when it is destroyed, production diminishes together with the oxygen demand for it (3-4,7).
PFC Is considered to be the treatment of choice to eliminate the angiogenic stimuli (2,9), although other retinal ablation treatments can be utilized such as cryotherapy or trans-scleral diode laser when the visualization is poor (9). It is also possible to perform pars plana vitrectomy with endophotocoagulation (3).
The low number of NVG secondary to inflammatory pathology should be treated with anti-inflammatory agents (2,9).
When the angiogenic stimuli is removed, the neovessels could regress and IOP levels become normalized in cases in the stage before the angle closure (2,6,8).
For patients with NVG secondary to DR with clear media, intense PFC is recommended with 1200-1600 impacts (2).
In the case of NVG secondary to CRVO, the CVOS recommends performing PFC only if INV or NVA appear with an extension of 2 or more hours, with close follow-up of patients to search of these signs. Hayreh states that PFC diminishes INV but does not find differences between NVA and NVG. In addition, said author warns that the treatment with PFC produces peripheral visual field reduction in an eye with central CV which is already damaged by CRVO (9,13,14).
In the group of patients with NVG secondary to IOS, treatment is more controversial. Typically PFC has been utilized. However, angiographic retinal ischemia is found infrequently against the high frequency of uveal ischemia. Accordingly, it seems recommendable to utilize PFC only for cases with angiographically demonstrated retinal ischemia (9). Etiological treatment with endartherectomy or carotid anastomosis seem to stabilize the condition and improve VA on some occasions, particularly in precocious cases (9,12). It is crucial to diminish IOP as much as possible as well as avoiding systemic hypotension (particularly nocturnal) to prevent the progression of the glaucomatous neuropathy and the expression of RCAO and AION due to the vascular commitment existing in the IOS (9,17) (fig. 3).
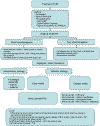
Fig. 3: Action protocol [Based on author's experience adapting schema by de
Sivak-Callcott JA (2)].
2. Medical treatment of neovascular glaucoma
In addition to the causal treatment of ischemia, various drugs must be applied to reduce IOP.
Hypotensor drugs: the drugs that reduce aqueous humor production are particularly recommended. These include topical betablockers, topical alpha-2 agonists and topical or systemic carbonic anhydrase inhibitors (CAI). Prostaglandins should be avoided as they exacerbate ocular inflammation and myotics as they diminish uveo-scleral flow (with a paradoxical effect of raising IOP) in addition to enhancing the formation of peripheral synechiae and altering the blood-aqueous barrier (2,19).
Anti-inflammatory and cyclopegic drugs: steroids control inflammation while cyclopegics diminish pain by inhibiting ciliar spasm, congestion and posterior synechiae (3,19).
All the above drugs must be associated so that a correct administration will comprise (3):
Betablockers or alpha-2 agonist or topical CAI + topical steroid + cyclopegic.
Fixed combinations of the above hypotensors can be utilized if necessary to implement their hypotensive action, facilitate patient compliance and minimize risks for the ocular surface.
A precise knowledge of the cardio-respiratory condition of the patient is crucial. Good anamnesis and positive interaction with family physicians is recommendable to control drug interactions, avoiding risks derived from the use of betablockers and other pharmacological groups (topical alpha-2 agonists and systemic CAI) (3,19).
Should it be necessary to utilize systemic CAI to achieve a higher hypotensive effect, these should not be associated to the use of topical drugs to avoid saturation of specific receptors. In addition, systemic CAI should be supplemented with potassium in order to prevent hydroelectrolytic alterations (3,19).
3. The role of anti-VEGF in the treatment of NVG
Anti-VEGF drugs have been utilized for compassionate purposes for treating NVG, both to reduce neovascular proliferation and modulate scarring in glaucoma surgery. NVG appears due to retinal-choroidal ischemia which increases the synthesis of VEGF and stimulates fibrovascular proliferation. The use of anti-VEGF drugs diminishes existing concentrations of VEGF and thus induce a regression of INV and NVA, allowing for improved IOP control before a synechial angle closure occurs (4,6-8,20-23). In addition, it is important to allow time for the base disease treatment (generally with PFC) to diminish the permanent synthesis of VEGF (4,6-8,24-27).
Recent studies have demonstrated that the use of intravitreal Bevacizumab (1.25 mg/0.05 ml) produces a regression of neovessels and improves IOP control, although recurrences seem to appear which respond to reinjection (8,26,28-30). However, in advanced NGV cases with angle closure, the regression of neovessels is not followed by enhanced IOP control. The use of coadjuvant anti-VEGF in glaucoma surgery improves the surgical prognosis by modulating scarring.
The prevalence of side effects include systemic effects (acute myocardium infarct, cerebrovascular event) and ocular effects (endophthalmitis, retina detachment) derived from intra-ocular use are minimum if administered in prescribed doses (25,28,31).
There is a lack of anti-VEGF preferred administration pathways although the following pathways have been utilized: topical, subconjunctival, sub-tenon, intra-chamber and intravitreal, the latter being the most widely and utilized pathway (20,31-35). Intra-ocular administration seems adequate for treating INV and NVA, whereas surface administration has been suggested as adjuvating treatment in surgeries to modulate scarring (31,32). The intra-ocular use in filtrating surgery and drainage implants also improves the prognosis by diminishing the risk of fibrovascular proliferation (6,25,31,32,36,37).
4. Surgical treatment
The selection of treatment must depend on the anatomic and functional condition of the ocular globe. There are two different options that can be applied: either filtrating procedures (trabeculectomy) or drainage devices, in order to increase the ease of exit of the aqueous humor, and cyclodestructive procedures that reduce the production thereof. The effectiveness of filtrating procedures and drainage devices is inferior to other pathologies (2,9).
Trabeculectomy: It can be performed if there is a good visual prognosis, iridian neovascularization (INV) is inactive, there is no synechial angle closure and IOP cannot be controlled with medical treatment. The surgery is more successful when the angiogenic stimuli is reduced with PFC.
The association of trabeculectomy with antimetabolites such as mitomycin C, at doses between 0.2-0.4 mg/ml with 2-4 minute exposures is more efficient to diminish the risk of fibrosis-induced failure. It is also possible to associate Bevacizumab instead of Mitomycin C or to use both during the subconjunctival applied surgical technique. In turn, the use of intra-chamber or intravitreal anti-VEGF improves the effectiveness of trabeculectomy, reinjecting in the post-ophthalmological if necessary (2,9,36-41).
Trabeculectomy can be associated with direct cauterization of the peripheral iris, which seems to reinforce its efficacy and minimize complications. Also, it is possible to combine with pars plana vitrectomy (PPV) as it seems to improve the effectiveness of the technique (which diminishes with time) (2,9,36-41).
Trabeculectomy can be associated with lens removal if the ocular globe conditions, patient visual requirements or the need to complete de retinal ablation treatment with PFC make it advisable (2,9,38).
The risk of failure in NGV trabeculectomy is high but it is also high with drainage devices and cycloablation treatments. Thus, the treatment of choice is yet to be determined. What is demonstrated is the improved prognosis with inactivity of neovascularization, which is achieved with anti-VEGF treatment. In our experience, trabeculectomy does not yield sufficiently good results and therefore we habitually apply drainage devices in the first approach (2,6,9,31,32,39).
Drainage devices: these are useful to control IOP, when there is visual function in patients with inactive INV (although the post-ophthalmological bleeding increases) and in resistant cases where conventional filtrating surgery has failed (2,9,42-46).
The perfect location is not in the anterior chamber (due to the danger of tube rotation and displacement, causing endothelial contact), and so we must search a site in the posterior chamber, performing the cataract extraction (if the patient is not pseudophakic) in the same surgical action. Another option is to perform pars plana vitrectomy which will allow us to place the tube in the vitreal chamber (3) (this procedure should be considered particularly in diabetic retinopathy). In our experience, this has given improved hypotensor and visual results.
It is highly advisable to associate surgery with anti-VEGF injection, either in anterior chamber or in vitreous, to avoid bleeding and surgery failure due to active neovascularization. The success of surgery diminishes as of the first year and failure is correlated to the persistence of INV. Accordingly, antiangiogenic should be injected if necessary (6,39,47-48).
There are no clear differences in surgery success with different implants. The success rate is higher if PPV combined surgery is associated to drainage tube in pars plana (49).
Cyclodestructive procedures
Diode laser cyclophotocoagulation and cryotherapy have been used when the patient visual function is poor. These procedures reduce the formation of aqueous humor through partial destruction of the ciliary body. Trans-scleral cyclophotocoagulation with diode laser produces less complications, pain and inflammation than cryotherapy. In addition, it exhibits the advantage of greater treatment simplicity. Therefore, the practice of cyclocryotherapy has been virtually discontinued. The most common complications are hyphema and hypotonia. If available, a further option is endoscopic cyclophotocoagulation (50,51).
The possibility of phthisis bulbi should not be discarded. It appears more frequently with cyclo-cryotherapy and when utilizing the Nd:YAG laser instead of diode laser for cyclophotocoagulation. However, as the natural course of the disease evolves in many cases towards phthisis bulbi, it is difficult to assess the extent in which the procedure influences the development of this complication (9).
The use of cyclophotocoagulation associated to anti-VEGF increases the reducing effect of IOP and diminishes neovascularization (52).
Palliative surgery in blind eye with pain
With the use of cyclophotocoagulation, nowadays it is rarely necessary to apply more aggressive procedures such as retrobulbar alcohol injection, evisceration or enucleation (9).
CONCLUSIONS
In this updated review on NVG we have endeavored to combine existing knowledge with the latest advances in anti-VEGF therapy. The protocols proposed in this review are based on scientific evidence and our own experience, and we believe that the application thereof in the treatment of NVG could improve its prognosis.
REFERENCES
-
Mocanu C, Barascu D, Marinescu F. Neovascular glaucoma-Retrospective study. Ophthalmologica 2005; 49: 58-65.
-
Sivak-Callcott JA, O’Day DM, Gass JD, Tsai JC. Evidence-based recommendations for the diagnosis and treatment of neovascular glaucoma. Ophthalmology 2001; 108: 1767-1776.
-
Kim D, Singh A, Annapurna S. Neovascular Glaucoma. En: Shaarawy TM. Glaucoma Medical Diagnosis and Therapy. Saint Louis (EEUU): Saunders Elsevier; 2009; I: 409-417.
-
Tripathi RC, Li J, Tripathi BJ, Chalam KV, Adams AP. Increased level of vascular endothelial growth factor in aqueous humor of patients with neovascular glaucoma. Ophthalmology 1998; 105: 232-237.
-
Ho QT, Kuo CJ. Vascular Endothelial Growth Factor: Biology and Therapeutic Applications. Int J Biochem Cell Biol 2007; 39: 1349-1357.
-
Horsley MB, Kahook MY. Anti-VEGF therapy for glaucoma. Curr Opin Ophthalmol 2010; 21: 112-117.
-
Aiello LP, Avery RL, Arrigg PG. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994; 331: 1480-1487.
-
Kohno RI, Hata Y, Mochizuki Y, Arita R, Kawahara R, Kita T, Miyazaki M, Hisatomi T, Ikeda Y, Aiello LP,Ishibashi T. Histopathology of Neovascular Tissue From Eyes With Proliferative Diabetic Retinopathy After Intravitreal Bevacizumab Injection. Am J Ophthalmol 2010; 150: 223-229.
-
Hayreh SS. Neovascular glaucoma. Prog Retin Eye Res 2007; 26: 470-485.
-
Sharma A, D’Amico DJ. Medical and surgical management of central retinal vein occlusion. Int Ophthalmol Clin 2004; 44: 1-16.
-
Haymore JG, Mejico LJ. Retinal vascular occlusion syndromes. Int Ophthalmol Clin 2009; 49: 63-79.
-
Ryan SJ. Retina. Fourth edition. Baltimore (EEUU): Elsevier; 2006; II: 1339-1354.
-
The Central Vein Occlusion Study Group. Arch Ophthalmol 1997; 115: 486-491.
-
Branch vein Oclussion Study Group. Argon laser scatter photocoagulation for prevention of neovascularization and hemorrhage in branch vein occlusion. Arch Ophthalmol 1986; 104: 34-41.
-
Ryan SJ. Retina. Fourth edition. Baltimore (EEUU): Elsevier; 2006; II: 1323-1338.
-
Ryan SJ. Retina. Fourth edition. Baltimore (EEUU): Elsevier; 2006; II: 1285-1322.
-
Mendrinos E, Machinis TG, Pournaras CJ. Ocular ischemic syndrome. Surv Ophthalmol 2010; 55: 2-34.
-
Ryan SJ. Retina. Fourth edition. Baltimore (EEUU): Elsevier; 2006; II: 1491-1502.
-
European Glaucoma Society. Terminología y Pautas para el Glaucoma. Third edition. Savona (Italia): Dogma; 2009.
-
Davidorf FH, Mouser JG, Derick RJ. Rapid improvement of rubeosis iridis from a single Bevacizumab (Avastin) injection. Retina 2006; 26: 354-356.
-
Mason JO 3rd, Albert MA Jr, Mays A, Vail R. Regression of neovascular iris vessels by intravitreal injection of Bevacizumab. Retina 2006; 26: 839-841.
-
Silva Paula J, Jorge R, Alves Costa R, Rodriguez Mde L, Scott IU . Short-term results of intravitreal bevacizumab (Avastin) on anterior segment neovascularization in neovascular glaucoma. Acta Ophthalmol Scand 2006; 84: 556-557.
-
Grisanti S, Biester S, Peters S,Tatar D, Ziemssen F, Bartz-Schmidt KU. Tuebingen Bevacizumab study group. Intracameral bevacizumab for iris rubeosis. Am J Ophthalmol 2006; 142: 158-160.
-
Ehlers JP, Spirn MJ, Lam A, Sivalingam A, Samuel MA, Tasman W. Combination intravitreal bevacizumab/panretinal photocoagulation versus panretinal photocoagulation alone in the treatment of neovascular glaucoma. Retina 2008; 28: 696-702.
-
Wakabayashi T, Oshima Y, Sakaguchi H, Ikuno Y, Miki A, Gomi F, Otori Y, Kamei M, Kusaka S, Tano Y. Intravitreal bevacizumab to treat iris neovascularization and neovascular glaucoma secondary to ischemic retinal diseases in 41 consecutive cases. Ophthalmology 2008; 115: 1571-1580.
-
Moraczewski AL, Lee RK, Palmberg PF, Rosenfeld PJ, Feuer WJ. Outcomes of treatment of neovascular glaucoma with intravitreal bevacizumab. Br J Ophthalmol 2009; 93: 589-593.
-
Fernandez-Vigo J, Castro J, Macarro A. Diabetic iris neovascularization. Natural history and treatment. Acta Ophthalmol Scand 1997; 75: 89-93.
-
Iliev ME, Domig D, Wolf- Schnurrbursch U, Wolf S,Sarra GM. Intravitreal bevacizumab (Avastin) in the treatment of neovascular glaucoma. Am J Ophthalmol 2006; 142: 1054-1056.
-
Costagliola C, Cipollone U, Rinaldi M, della Corte M, Semeraro F, Romano MR. Intravitreal bevacizumab (Avastin) injection for neovascular glaucoma: A survey on 23 cases throughout 12-month follow-up. Br J Clin Pharmacol 2008; 66: 667-673.
-
Saito Y, Higashide T, Takeda H, Murotani E, Ohkubo S, Sugiyama K. Clinical Factors Related to Recurrence of Anterior Segment Neovascularization After Treatment Including Intravitreal Bevacizumab. American Journal of Ophthalmology 2010; 149; 6: 964-972.
-
Martínez-Carpio PA, Bonafonte-Márquez E, Heredia-García CD, Bonafonte-Royo S. Efficacy and safety of intravitreal injection of bevacizumab in the treatment of neovascular glaucoma. Arch Soc Esp Oftalmol 2008; 83: 579-588.
-
Gunther JB, Altaweel MM. Bevacizumab (Avastin) for the Treatment of Ocular Disease. Survey of Ophthalmology 2009; 54: 372-400.
-
Avery RL. Regression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin) treatment. Retina 2006; 26: 352-354.
-
Vasudev D, Blair MP, Galasso J, Kapur R, Vajaranant T. Intravitreal bevacizumab for neovascular glaucoma. J Ocul Pharmacol Ther 2009; 25: 453-458.
-
Gupta V, Jha R, Rao A, Kong G, Sihota R. The effect of different doses of intracameral bevacizumab on surgical outcomes of trabeculectomy for neovascular glaucoma. Eur J Ophthalmol 2009; 19: 435-41.
-
Allen RC, Bellows AR, Hutchinson BT, Murphy SD. Filtration surgery in the treatment of neovascular glaucoma. Ophthalmology 1982; 89: 1181-1187.
-
Grewal DS, Jain R, Kumar H, Grewal SS. Evaluation of Subconjunctival Bevacizumab as an Adjunct to Trabeculectomy. Ophthalmology 2008; 115: 2141-2145.
-
Vasconcelos de Moraes CG, Facio Jr AC, Costa JH,Santiago Malta RF. Intracameral Bevacizumab and mitomycin C Trabeculectomy for eyes with neovascular glaucoma a cases series. J Ocul Biol Dis Inform 2009; 2: 40-46.
-
Ichhpujani P, Ramasubramanian A, Kaushik S, Pandav SS. Bevacizumab in glaucoma. Can J Ophthalmol 2007; 42: 812-815.
-
Elgin U, Berker N, Batman A. Trabeculectomy with mitomycin C combined with direct cauterization of peripheral iris in the management of neovascular glaucoma. J Glaucoma 2006; 15: 466-470.
-
Takihara Y, Inatani M, Fukushima M, Iwao K, Iwao M, Tanihara H. Trabeculectomy with Mitomycin C for Neovascular Glaucoma: Prognostic Factors for Surgical Failure. Am J Ophthalmol 2009; 147: 912-918.
-
Sidoti PA, Dunphy TR, Baerveldt G, Experience with the Baerveldt glaucoma implant in treating neovascular glaucoma. Ophthalmology 1995; 102: 1107-1118.
-
Every SG, Molteno AC, Bevin TH, Herbison P, Long-term results of Molteno implant insertion in cases of neovascular glaucoma. Arch Ophthalmol 2006; 124: 355-360.
-
Mermoud A, Salmon JF, Alexander P. Molteno tube implantation for neovascular glaucoma: Long-term results and factors influencing the outcome. Ophthalmology 1993; 100: 897-902.
-
Rush R. Ciliary sulcus Ahmed Glaucoma Valve tube placement in neovascular glaucoma. Ophthalmic Surg Lasers Imaging 2009; 40: 489-492.
-
Minckler DS, Francis BA, Hodapp EA, Jampel HD, Lin SC, Samples JR, Smith SD, Singh K. Aqueous Shunts in Glaucoma. Ophthalmology 2008; 115: 1089-1098.
-
Eid TM, Radwan A, el-Manawy W, el-Hawary I. Intravitreal bevacizumab and aqueous shunting surgery for neovascular glaucoma: safety and efficacy. Can J Ophthalmol 2009; 44: 451-456.
-
Mahdy RA. Adjunctive Use of Bevacizumab Versus Mitomycin C With Ahmed Valve Implantation in Treatment of Pediatric Glaucoma. J Glaucoma 2010; 16.
-
Netland P. The Ahmed Glaucoma Valve in Neovascular Glaucoma (An AOS Thesis). Trans Am Ophthalmol Soc 2009; 107: 325-342.
-
Iliev ME, Gerber S. Long-term outcome of trans-scleral diode laser cyclophotocoagulation in refractory glaucoma. Br J Ophthalmol 2007; 91: 1631-1635.
-
Delgado MF, Dickens CJ, Iwach AG, Novack GD, Nychka DS, Wong PC, Nguyen N. Long-term results of noncontact neodymium:yttrium-aluminum-garnet cyclophotocoagulation in neovascular glaucoma. Ophthalmology 2003; 110: 895-899.
-
Ghosh S, Singh D, Ruddle JB, Shiu M, Coote MA, Crowston JG. Combined diode laser cyclophotocoagulation and intravitreal bevacizumab (Avastin) in neovascular glaucoma. Clin Exp Ophthalmol 2010; 38; 4: 353-357.