UPDATE REVIEW
Refractive defects in childhood (I)
GRABOWSKA A1, NOVAL S2, VILLAFRANCA HOLGUÍN M3, GRANADOS FERNÁNDEZ M4, PERALTA J5
1
Graduate in Medicine. Ophthalmology Service. La Paz University Hospital/Autonomous
University of Madrid. IdiPAZ Research Institute.
2 Ph.D. in Medicine. Pediatric Ophthalmology Service. La Paz
University Hospital/Autonomous University of Madrid. IdiPAZ Research Institute.
3 Optometrician. Vissum Ophthalmological Corporation. Madrid.
4 Graduate in Medicine. Pediatric Ophthalmology Service. La Paz
University Hospital/Autonomous University of Madrid. IdiPAZ Research Institute.
5 Ph.D. in Medicine. Pediatric Ophthalmology Service. La Paz
University Hospital/Autonomous University of Madrid. IdiPAZ Research Institute.
ABSTRACT
Alterations in the emmetropization process can give rise to the development of refractive defects such as myopia, hypermetropia and astigmatism. Research suggests that genetic factors play a crucial role in the development of myopia and hypermetropia. However, there is evidence about the influence of environmental factors. The role of genetic factors in the development of astigmatism is controversial. This review presents the definition, prevalence and etiopathogeny of refractive errors in the pediatric population.
INTRODUCTION
Emmetropization is considered to be a coordination process involving the power of the cornea, the lens and the axial length, with the input of visual stimuli, so that in the adult age the focal point will impinge on the plane of the retina. The mean refractive defect of 3 month-old infants is of +2.16 diopters (D), with a standard deviation (SD) OF 1.30 (1). The currently most accepted model considers that poor focus caused by this hypermetropic defect stimulates ocular growth in order to reduce hypermetropia (1). The mean axial length when reaching emmetropia in adult age is of 23.6 mm (SD 0.7 mm)2.
Between month 6 and 12, the increased axial length is offset with changes at the anterior pole level. The cornea diminishes its reflective power and increases its radius, while the anterior chamber goes from a depth of 2.4 mm at birth to 3.5 mm at month 12, giving rise to an increased distance between the cornea and the lens which translates in an overall refractive power reduction of 0.8. After the first year and up to the end of emmetropization, adjustments are carried out mainly in the lens, with the anterior radius going from 5 mm to 10 mm and to the posterior radius from 4 to 6 mm at the end of emmetropization. Generally, emmetropia is reached between age 9 and 14 (1,3).
Two theoretical emmetropization mechanisms have been described: one is passive, under genetic control, and the other is active under environmental control. The active emmetropization model caused by visual feedback assumes that the eye regulates its growth in response to stimuli produced by focal length errors, in an attempt to reduce said errors. At present, three active emmetropization mechanisms have been described: by deprivation (4,5), by loss of focus (6), and by drugs (7). The deprivation mechanism is observed after the occlusion of one eye, and the loss of focus mechanism by the aposition of a negative lens in the visual axis.
Both mechanisms regulate scleral growth, increasing the axial length of the ocular globe, mainly regulated by the collinergic system. The drug-induced mechanism can vary. In the case of topiramate (8), it seems related to its inhibiting effect on carbonic anhydrase and induction of cilio-choroidal effusion.
An alteration in the emmetropization process can cause the development of refractive defects such as myopia, hypermetropia and astigmatism. In children, refractive error must be measured under cycloplegia to avoid an overestimation of myopia or underestimation of hypermetropia due to its considerable accommodation capacity (9).
MIYOPIA
Simple myopia is a spherical ammetropia, i.e., that the refractive error is equal in all corneal meridians where the parallel rays coming from the infinite, instead of focalizing on the retina, focus in front of it.
The prevalence of myopia differs in published series according to its definition, the population being studied and the method utilized for refraction. The prevelance of myopia in the first year of life has been estimated between 4% and 5% (10) and increases progressively with age. The CLEERE (Collaborative Longitudinal Evaluation of Eth¬nicity and Refractive Error) (11) study on US children, which included 2523 children between 5 and 17, indicated that the prevalence of myopia was of 9.2%. Said prevalence was greater in Asian children (18.5%) followed by Latin American children (13.2%) and Africans (6.6%). The lowest prevalence was found in Caucasian children (4.4%). The results of the Pediatric Refractive Study in India (12), Chile (13), Africa (14), Malaysia (15) and China (16,17) confirmed that a low prevalence (<5%) between age 5 and 6 increases significantly up to 15 years of age. It emphasized the high prevalence (>35%) of myopia in the older and female Chinese population.
Multiple studies have demonstrated that myopization has a genetic base. Studies on twins have observed a high rate of inheritance of refractive defects and all involved components (axial length, corneal curvature and lens power). Nine loci related to myopia have been detected, including seven with dominant ausotomic inheritance pattern (21q22.3, 18p11.31, 12q21-q23, 7q36, 17q21-q22, 4q22-q27, 2q37.1), one autosomic recessive (14q22.1- q24.2) and one recessive linked to chromosome X (Xq28, Xq23-q25) (18,19). Experimental studies indicate that these genes expressed cytokines, neurotransmitters, scleral matrix proteins, and vitreous and eye growth regulators (20-22). Although the actual parallelism between myopia induced in animals with human physiological myopia, animal studies lead to a fundamental conclusion: that emmetropization is a genetically controlled process even though it depends on the visual environment.
In one third of cases there is evidence about the role of the environment in the development of myopia (23). In the past it was suggested that exposure to artificial light during the first 2 years of life constituted an important pathogenic factor. The study by Quinn et al (24) revealed that 10% of children who slept without artificial lighting, 34% who slept with a night lamp and 55% of children who slept with artificial light developed myopia. The research by Gwiazda et al (25) and Zadnick et al (26) did not confirm these results, which could suggest that nocturnal artificial lighting could have some protective effect. The differences between studies, such as the mean age of children, could influence results. Additional research is required to clarify this.
Reading habits and other near sight activities seem unrelated to the risk of developing myopia in children under 6 (27). However, these habits do seem to have an influence in children above that age. The SCORM study (Singapore Cohort Study of the Risk Factors for Myopia) (28), which assessed children between 7 and 9, concluded that the children who read more than 2 books a week had a higher probability of developing myopia above 3.0 D. An additional study carried out in the Chinese children population of Singapore and Sydney demonstrated a correlation between diminished outdoor activities and the risk of developing myopia (29). The differences between the rural environment and urban areas confirmed that the appearance of myopia is influenced by environmental factors (30).
Lower weight at birth and the severity of premature retinopathy has been associated with higher risk of myopia. Twenty percent of lactating babies with very low weight at birth (under 1,251 g) exhibit myopia in the first 2 years of life and 4.6% exhibit a severe type of myopia (>5.0 D) (31). The ETROP study (Early Treatment for Retinopathy of Prematurity) (32) observed how a 48.4% risk of developing myopia in premature retinopathy cases that resolved without treatment increased to 60.6% of cases treated at the pre-threshold high risk stage and to 80.9% in eyes treated at the threshold stage.
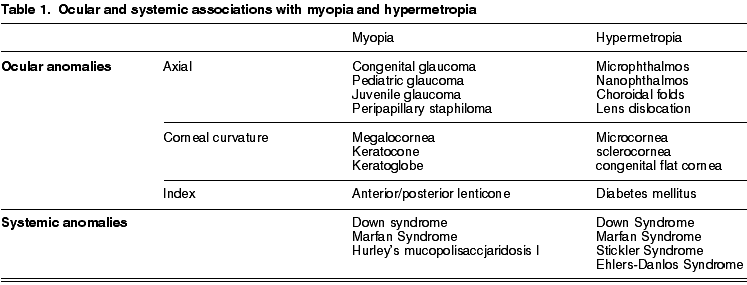
Finally, myopia has been associated to some ocular anomalies and systemic diseases (table 1) due to changes in the corneal curvature, in the lens refraction index or in the ocular axial length. For example, in the Marfan syndrome two possible responsible mechanisms have been described: anterior migration of the irido-lens diaphragm that anteriorly displaces the focal point of the eye and the lens thickening that increases its refractive power (33). Alterations of the elastic component of the sclera and the cornea could also cause an increase of the axial length and therefore myopia (34). A similar mechanism would account for the development of myopia in other connective tissue diseases.
HYPERMETROPIA
This is a spherical ametropia where theoretically parallel light rays coming from a far object are refracted in such a way that they converge at a point behind the plane of the retina. The most frequent cause is a reduction of the anterior-posterior axis of the eye (axial hypermetropia) of genetic origin. However, there are other types of hypermetropias (due to curvature, index and alteration of the lens position) (table 1).
Slight hypermetropia is a physiological condition in the pediatric population. In contrast with myopia, hypermetropia usually diminishes with age. Mutti et al (1) observed in a group of 221 3-month old babies that 24.8% exhibited a cycloplegic refraction of at least 3.0 D of hypermetropia. When they were 9 months old, the prevalence has diminished to 5.4% (35). In the CLEERE study (11), the prevalence of hypermetropia also diminished with age. However, other studies indicate an increase of hypermetropia, up to age 7 (36,37). As for the prevalence of hypermetropia (at least +1.0 D) in different ethnic groups, by order of frequency it is greater in Caucasian children (32.4%) than in Latin American children (20.7%), Afro-American children (12.2%) and Asian children (12.0%) (11).
Various studies have analyzed the prevalence of high hypermetropia and its importance in the development of abnormal visual function (38,39). The data of the Mutti et al study (1) suggest that children with high hypermetropia (>5.0 D) do not achieve emmetropization, against those with low hypermetropia who usually become emmetropic.
At present it is considered that genetic factors play a crucial role in the development of hypermetropia. Studies demonstrate a higher inheritance index of hypermetropia (1-7 D) in monozygotic than in dizygotic twins (40). Population studies also exhibit familial aggregation (41). In a population of 34 newborn babies having a parent or a brother with endotropia, when they reached 6 months of age 38% exhibited at least 4 D of hypermetropia, which exceeds the prevalence for the general population at the same age (42). Inheritance is different according to the type of hypermetropia. In weak hypermetropiae up to 6 D, the inheritance pattern is autosomic dominant. In high hypermetropia, inheritance is autosomic recessive and sometimes is related with ocular and general malformations.
It has recently been suggested that the risk of developing hypermetropia is associated to childhood growth. Accordingly, any delay in general growth would interrupt the emmetropization process, giving rise to short axial length (43). This could explain the high prevalence of hypermetropia in children with growth hormone deficiency (44).
ASTIGMATISM
In astigmatism, light rays paralell to the eye do not meet at the same image point but on two focal lines perpendicular to each other (regular astigmatism) or without defined focus points (irregular astigmatism). This is due to the irregular or thoric surface of the cornea and lens. In childhood, astigmatism is predominantly corneal. Newborns have pointed and astigmatic corneae (generally against the rule) (fig. 1). Lens ectopia in Marfan syndrome is an example of non-corneal astigmatism. The greater prevalence of astigmatism has been observed in the first year of life (45), particularly in newborns with lower weight at birth and lower gestational age (46,47). The development of the ocular globe tends to correct astigmatism in the first years of life. The CLEERE study (11) indicated that the prevalence of astigmatism (a difference between the two corneal meridians of at least 1.0 D) also differed according to the ethnic type, being of 20% in Afro-Americans, 33.6% in Asians, 36.9% in Latin Americans and of 26.4% in Caucasian children.
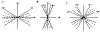
Fig. 1: A: Reverse or against the rule astigmatism: the maximum power Meridian
is the horizontal one (between 30º and 150º), B: Direct or as per the rule
astigmatism: the maximum power Meridian is the vertical one (between 60º and
120º), C: Oblique astigmatism: when one of the main meridians is at 45º (±15º)
and the other at 135º (±15º).
The influence of genetic factors is controversial. Teikari et al. (48) did not find differences between monozygotic and dizygotic twins, which would reduce the influence of genetic factors vis-à-vis environmental factors in the development of astigmatism. However, the results on the research on twins carried out by Hammond et al (49) demonstrated the opposite. Clement et al (50) analyzed data of 125 families affected by astigmatism and described a dominant autosomic inheritance pattern.
Any pathology which can produce malformations in the cornea, such as chalazion or pterygion, can induce astigmatism by altering the relationship between corneal meridians. Robb et al (51) described increases in astigmatism time in 16 of the 37 children with palpebral and orbitary hemangiomas.
Ptosis and ptosis surgery have also been described as predisposing factors for astigmatism. Patients with congenital ptosis have a higher degree of corneal topographic asymmetry as well as a higher degree of astigmatism (52). Howland et al (53) suggested that corneal astigmatism could be the result of unequal forces exerted by extraocular muscles on the cornea. In addition, the stress induced by the eyelids has been proposed as a possible factor in the development of corneal astigmatism. This would explain the high prevalence of astigmatism in Down syndrome and the increase of its power over time (54). The greater prevalence of oblique astigmatism supports this theory as the correlation between the astigmatism axis and the superior inclination of the temporal edge has been observed (55).
CONCLUSIONS
The exact cause of refractive defects is not yet known. Research carried out to date, mainly in twins, suggests that genetic factors play a crucial role in the development of myopia and hypermetropia. However, there is also evidence of the influence of environmental factors. The role of astigmatism is controversial. The interaction between eyelids and the tension of the extra-ocular muscles seems to explain the development of astigmatism in various ethnic groups and diseases.
REFERENCES
-
Mutti DO, Mitchell GL, Jones LA, Friedman NE, Frane SL, Lin WK, et al. Axial growth and changes in lenticular and corneal power during emmetropization in infants. Invest Ophthalmol Vis Sci 2005; 46: 3074-3080.
-
Gordon RA, Donzis PB. Refractive development of the human eye. Arch Ophthalmol 1985; 103: 785-789.
-
Wildsoet CF. Active emmetropization—evidence for its existence and ramifications for clinical practice. Ophthalmic Physiol Opt 1997; 17: 279-290.
-
Wiesel TN, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature 1977; 266: 66-68.
-
Smith EL 3rd, Hung LF. Form-deprivation myopia in monkeys is a graded phenomenon. Vision Res 2000; 40: 371-381.
-
Smith EL 3rd, Hung LF, Huang J, Blasdel TL, Humbird TL, Bockhorst KH. Effects of optical defocus on refractive development in monkeys: evidence for local, regionally selective mechanisms. Invest Ophthalmol Vis Sci 2010; 51: 3864-3873.
-
Metlapally S, McBrien NA. The effect of pirenzepine on positive- and negative-lens-induced refractive error and ocular growth in chicks. Invest Ophthalmol Vis Sci 2010; 51: 5438-5444.
-
Fraunfelder FW, Fraunfelder FT, Keates EU. Topiramate-associated acute, bilateral, secondary angle-closure glaucoma. Ophthalmology 2004; 111: 109-111.
-
Mutti DO, Zadnik K, Egashira S, Kish L, Twelker JD, Adams AJ. The effect of cycloplegia on measurement of the ocular components. Invest Ophthalmol Vis Sci 1994; 35: 515-527.
-
Atkinson J, Braddick OJ, Durden K, Watson PG, Atkinson S. Screening for refractive errors in 6-9 month old infants by photorefraction. Br J Ophthalmol 1984; 68: 105-112.
-
Kleinstein RN, Jones LA, Hullett S, Kwon S, Lee RJ, Friedman NE, et al. Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error Study Group. Refractive error and ethnicity in children. Arch Ophthalmol 2003; 121: 1141-1147.
-
Murthy GV, Gupta SK, Ellwein LB, Muñoz SR, Pokharel GP, Sanga L, et al. Refractive error in children in an urban population in New Delhi. Invest Ophthalmol Vis Sci 2002; 43: 623-631.
-
Maul E, Barroso S, Munoz SR, Sperduto RD, Ellwein LB. Refractive error study in children: results from La Florida, Chile. Am J Ophthalmol 2000; 129: 445-454.
-
Naidoo KS, Raghunandan A, Mashige KP, Govender P, Holden BA, Pokharel GP, et al. Refractive error and visual impairment in African children in South Africa. Invest Ophthalmol Vis Sci 2003; 44: 3764-3770.
-
Goh PP, Abqariyah Y, Pokharel GP, Ellwein LB. Refractive error and visual impairment in school-age children in Gombak District, Malaysia. Ophthalmology 2005; 112: 678-685.
-
Zhao J, Pan X, Sui R, Munoz SR, Sperduto RD, Ellwein LB. Refractive error study in children: results from Shunyi District , China. Am J Ophthalmol 2000; 129: 427-435.
-
He M, Zeng J, Liu Y, Xu J, Pokharel GP, Ellwein LB. Refractive error and visual impairment in urban children in Southern China. Invest Ophthalmol Vis Sci 2004; 45: 793-799.
-
Montero JA, Ruiz-Moreno JM. Fisiopatología y clínica de la patología macular miópica. En: Armadá Maresca F. Patología y cirugía de la mácula. Madrid: Sociedad Española de Oftalmología; 2010; 805-807.
-
Hornbeaka DM, Young TL. Myopia genetics: a review of current research and emerging trends. Curr Opin Ophthalmol 2009; 20: 356-362.
-
Ashby RS, Feldkaemper MP. Gene expression within the amacrine cell layer of chicks after myopic and hyperopic defocus. Invest Ophthalmol Vis Sci 2010; 51: 3726-3735.
-
Frost MR, Norton TT. Differential protein expression in tree shrew sclera during development of lens-induced myopia and recovery. Mol Vis 2007; 13: 1580-1588.
-
Halfter W, Winzen U, Bishop PN, Eller A. Regulation of eye size by the retinal basement membrane and vitreous body. Invest Ophthalmol Vis Sci 2006; 47: 3586-3594.
-
Lopes MC, Andrew T, Carbonaro F, Spector TD, Hammond CJ. Estimating heritability and shared environmental effects for refractive error in twin and family studies. Invest Ophthalmol Vis Sci 2009; 50: 126-131.
-
Quinn GE, Shin CH, Maguire MG, Stone RA. Myopia and ambient lighting at night. Nature 1999; 399: 113-114.
-
Gwiazda J, Ong E, Held R, Thorn F. Myopia and ambient night-time lighting. Nature 2000; 404: 144.
-
Zadnik K, Jones LA, Irvin BC, Kleinstein RN, Manny RE, Shin JA, et al. Myopia and ambient night-time lighting. CLEERE Study Group. Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error. Nature 2000; 404: 143-144.
-
Low W, Dirani M, Gazzard G, Chan YH, Zhou HJ, Selvaraj P, et al. Family history, near work, outdoor activity, and myopia in Singapore Chinese preschool children. Br J Ophthalmol 2010; 94: 1012-1016.
-
Saw SM, Chua WH, Hong CY, Wu HM, Chan WY, Chia KS, et al. Nearwork in early-onset myopia. Invest Ophthalmol Vis Sci 2002; 43: 332-339.
-
Rose KA, Morgan IG, Smith W, Burlutsky G, Mitchell P, Saw SM. Myopia, lifestyle, and schooling in students of Chinese ethnicity in Singapore and Sydney. Arch Ophthalmol 2008; 126: 527-530.
-
Ip JM, Rose KA, Morgan IG, Burlutsky G, Mitchell P. Myopia and the urban environment: findings in a sample of 12-year-old Australian school children. Invest Ophthalmol Vis Sci 2008; 49: 3858-3863.
-
Quinn GE, Dobson V, Repka MX, Reynolds J, Kivlin J, Davis B, et al. Development of myopia in infants with birth weights less than 1251 grams. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology 1992; 99: 329-340.
-
Davitt BV, Dobson V, Good WV, Hardy RJ, Quinn GE, Siatkowski RM, et al. Early Treatment for Retinopathy of Prematurity Cooperative Group. Prevalence of myopia at 9 months in infants with high-risk prethreshold retinopathy of prematurity. Ophthalmology 2005; 112: 1564-1568.
-
Dagi LR, Walton DS. Anterior axial lens subluxation, progressive myopia, and angle-closure glaucoma: recognition and treatment of atypical presentation of ectopia lentis. J AAPOS 2006; 10: 345-350.
-
Wheatley HM, Traboulsi EI, Flowers BE, Maumenee IH, Azar D, Pyeritz RE, et al. Immunohistochemical localization of fibrillin in human ocular tissues: relevance to the Marfan syndrome. Arch Ophthalmol 1995; 113: 103-109.
-
Tarczy-Hornoch K. The epidemiology of early childhood hyperopia. Optom Vis Sci 2007; 84: 115-23.
-
Slapter FJ. Age norms of refraction and vision. Arch Ophthalmol 1950; 43: 466-481.
-
Brown EVL. Net average yearly changes in refraction of atropinized eyes from birth to beyond middle life. Arch Ophthalmol 1938; 19: 719.
-
Pennie FC, Wood IC, Olsen C, White S, Charman WN. A longitudinal study of the biometric and refractive changes in full-term infants during the first year of life. Vision Res 2001; 41: 2799-2810.
-
Atkinson J, Anker S, Bobier W, Braddick O, Durden K, Nardini M, et al. Normal emmetropization in infants with spectacle correction for hyperopia. Invest Ophthalmol Vis Sci 2000; 41: 3726-3731.
-
Hammond CJ, Snieder H, Gilbert CE, Spector TD. Genes and environment in refractive error: the twin eye study. Invest Ophthalmol Vis Sci 2001; 42: 1232-1236.
-
Lee KE, Klein BE, Klein R, Fine JP. Aggregation of refractive error and 5-year changes in refractive error among families in the Beaver Dam Eye Study. Arch Ophthalmol 2001; 119: 1679-1685.
-
Aurell E, Norrsell K. A longitudinal study of children with a family history of strabismus: factors determining the incidence of strabismus. Br J Ophthalmol 1990; 74: 589-594.
-
Al-Bagdady M, Murphy PJ, Woodhouse JM. Development and distribution of refractive error in children with Down’s syndrome. Br J Ophthalmol 2010. doi:10.1136/bjo.2010.185827.
-
Parentin F, Tonini G, Perissutti P. Refractive evaluation in children with growth defect. Curr Eye Res 2004; 28: 11-15.
-
Howland HC, Sayles N. Photorefractive measurements of astigmatism in infants and young children. Invest Ophthalmol Vis Sci 1984; 25: 93-102.
-
Friling R, Weinberger D, Kremer I, Avisar R, Sirota L, Snir M. Keratometry measurements in preterm and full term newborn infants. Br J Ophthalmol 2004; 88: 8-10.
-
Isenberg SJ, Del Signore M, Chen A, Wei J, Christenson PD. Corneal topography of neonates and infants. Arch Ophthalmol 2004; 122: 1767-1771.
-
Teikari JM, O’Donnell JJ. Astigmatism in 72 twin pairs. Cornea 1989; 8: 263-266.
-
Hammond CJ, Sneider H, Gilbert CE, Spector TD. Genes and environment in refractive error: the twin eye study. Invest Ophthalmol Vis Sci 2001; 42: 1232-1236.
-
Clementi M, Angi M, Forabosco P, Di-Gianantonio E, Tenconi R. Inheritance of astigmatism: evidence for a major autosomal dominant locus. Am J Hum Genet 1998; 63: 825-830.
-
Plager DA, Snyder SK. Resolution of astigmatism after surgical resection of capillary hemangiomas in infants. Ophthalmology 1997; 104: 1102-1106.
-
Ugurbas SH, Zilelioglu G. Corneal topography in patients with congenital ptosis. Eye 1999; 13: 550-554.
-
Howland HC, Sayles N. Photokeratometric and photorefractive measurements of astigmatism in infants and young children. Vision Res 1985; 25: 73-81.
-
Haugen OH, Hovding G, Lundstrom I. Refractive development in children with Down’s syndrome: a population based, longitudinal study. Br J Ophthalmol 2001; 85: 714-719.
-
Gracia ML, Huang D, Crowe S, et al. Relationship between the axis and the degree of high astigmatism and obliquity of palpebral fissure. J AAPOS 2003; 7: 14-22.