TECHNOLOGICAL UPDATES IN OPHTHALMOLOGY
Fungal infection in a DALK interface: a new entity, a new approach
RodrÍguez Ausín P1, del RÍo Hermann E2, Hita AntÓn C1, MartÍn-RabadÁn p3, Peláez t3
1
Cornea and Ocular Surface Section. Torrejón Hospital. Torrejón de Ardoz, Madrid. Suárez-Leoz Clinic, La Milagrosa, Madrid.2 Ophthalmology Department. Gregorio Marañon General University hospital. Madrid.
3 Microbiology Service. Mycology Service. Gregorio Marañon General University hospital. Madrid.
Lamellar keratoplasty has multiple advantages over penetrating keratoplasty, including the avoidance of avoidance of intraocular innoculation of microorganisms during surgery, with the ensuing risk of endophthalmitis. Fungal infection of a DALK interface is an infrequent entity posing a diagnostic and therapeutic challenge. We introduce the topic reviewing general characteristics of fungal infections in penetrating and lamellar transplants, subsequently commenting our experience in the case of infection by
Candida guilliermondii after performing a DALK in a herpetic keratitis. We describe the technique developed for taking interface samples and the way in which intra-stromal treatment can control the infection without requiring hot penetrating keratoplasty.
I. FUNGAL INFECTIONS IN KERATOPLASTY
Candida infections after penetrating keratoplasty (PK) have frequently been described in the literature and are related to donor infections detected in surplus ring cultures after trephination. The risk of endophthalmitis after PK is estimated at 0.2%. However, if the donor ring culture is positive, it increases to 1% (1). Even so, this is controversial and some hospitals have discarded ring cultures because the frequency of endophthalmitis in negative cultures is nearly identical (1.3%) and in addition the microorganisms isolated from the ring and those of the vitreous not always match (2).
In the case of fungi the problem seems to be different: no fungal infections have been found with negative ring cultures and in addition the match rate between donor and receptor is of 100%. The incidence of fungal infection after PK was estimated at 0.16% and this frequency seems to have increased since the beginning of the current century compared against the previous decade (3).
Positive fungi ring cultures are infrequent but raise the risk to 14% (4). Prophylactic treatment has been suggested for cornea implant patients with positive cultures (4,5).
The clinic of Candida infection in PK comprises the appearance of white plates on the endothelium, usually close to the wound, with inflammatory reaction in the anterior chamber, hypopium, corneal edema and possible vitreous involvement. Its onset ranges between 10 hours and 5 months after PK (6). In 2009, Caldwell presented a case of post PK infection by
Candida glabrata and summarized the literature on the topic, finding that this species is on the rise. Of the 9 PK endophthalmitis cases by Candida glabrata, 5 were reported since 2006. There is a certain latency capacity confirmed by a patient contaminated by Candida glabrata 146 days post- surgery, with the condition remaining latent until then (5). By nature, it is resistant to treatment and recurrence has arisen in cases where it was believed that the infection had been eradicated but became activated spontaneously or in association to some event such as cataract surgery or corticoids treatment due to rejection (7,8).Even though the techniques to determine antifungal sensitivity are standardized, the choice of therapy in ocular infections continues to be empirical, lacking significant evidence on the correlation between the clinical response and in vitro sensitivity, particularly for intermediate CMI. On the other hand, there is very little information about the pharmaceutical activity of topical or local antifungal products in ophthalmology. A survey carried out in 2009 by Loh among 92 cornea specialists all over the world presented a broad variation in the management of fungal keratitis. For filament fungi, the most widely used treatment is Natamycin followed by Amphotericin and Voriconazole, whereas for yeasts the most widely used drug is Amphotericin followed by Natamycin and Voriconazole. In both cases, topical Voriconazole is considered to be the best drug although it is not widely used due to its high cost and the lack of supporting evidence. Half of the survey respondents utilized therapies combining several antifungal drugs. Systemic treatment is utilized at some point of the treatment by up to 92% of specialists: Voriconazole and itraconazole for filament fungii and Fluconazole and Voriconazole for yeasts, followed by itraconazole and ketoconazole.
The lack of definitive data on the efficacy of antifungal drugs may lead the clinician to apply multiple therapies in the hope of maximizing their benefits (9). Intrachamber injection of Amphotericin was successfully applied in deep mycosis which did not respond to conventional topical and oral treatment (10). In recent years, new azoles and echinocandines have been introduced in clinic although ocular experience is limited (11).
In most cases, treatment of post-keratoplasty fungal infection involves therapeutic penetrating keratoplasty with poor results due to the existence of associated endophthalmitis, although in recent years other therapeutic options have been reported. In 1998, Chapman resolved a case with repeated intrachamber Amphotericin injections which remained clear 2.5 years later (8). In 2005, Garcia Valenzuela presented a case of infection by
Candida glabrata due to donor contamination which coursed with recurring keratitis and endophthalmitis which was treated with intrastromal and intravitreal Amphotericin injections without recurrence after 18 months of follow-up (7). In 2006, Grueb reported a case of endophthalmitis also caused by Candida glabrata which was treated with 2 intrachamber 5-microgram Amphotericin injections and 0.1% eyedrops although the reported follow-up covered only 2 months (12). Local treatment, with greater drug availability, seems to be better than systemic treatment which, in the case of parenteral Amphotericin, involves complicated management and severe side effects. We must remember that the first case of Candida glabrata in keratoplasty died during treatment (13). There is no published experience with liposomal formulations in keratoplasty.In what concerns Voriconazole, there are numerous published cases of success for the treatment of fungal keratitis and endophthalmitis with oral, topical, intravitreal and intrastromal pathways. In 2008, in a literature review, Hariprasad concluded that
Voriconazole is an efficient and safe drug with good penetration and intraocular concentration (14). Its topical use in 1% eyedrops seems safe and well tolerated with good penetration in aqueous. Even so, more studies are necessary to determine if it is useful as first line and monotherapy as well as its best concentration and optimal dosage (15).
II. FUNGAL INFECTIONS IN LAMELLAR ANTERIOR KERATOPLASTY AND DSAEK
With the inclusion of lamellar techniques in daily practice, this pathology has begun to be described in partial thickness DSAEK (Descemet`s Stripping with Automated Endothelial Keratoplasty) and DALK. In both techniques the infection is located in a deep and difficult to access interface. A similar condition although more accessible is the post-LASIK fungal infection in which samples are taken by lifting the flap while washing. As this is probably a more superficial infection, topical treatment has proved to be efficient in published cases (16).
In August 2007, Fontana et al presented a
Candida albicans case in a patient with DALK due to keratocone, with positive donor ring culture and liquid medium for the fungus. Preventive treatment was not established and 4 weeks later the infiltrates appeared. The patient did not respond to topical and IV Amphotericin and even though the button was raised and replaced after irrigating the interface, infiltrates appeared 15 days later. A PPK (protected penetrating keratoplasty) had to be performed which after 6 months was transparent. Aqueous cultures at the time of the PPK were negative for fungi (16).In September of the same year, Kanabi et al presented 2 candidiasis cases after DALK due to keratocone, one
Candida glabrata and a furtherCandida albicans
in which the diagnostic was anatomopathologic after an initial diagnostic of epithelial endogrowth. In the first case, dotted infiltrates appeared at month 2 and PPK had to be performed due to rupture of Descemet’s membrane during the interface washing. The patient was treated with topical intra-chamber Amphotericin and oral Ketoconazol without relapse. Donor culture was not performed but the receptor of the opposite eye exhibited a clear graft in PPK.The other case debuted after 2.5 months and was initially treated with high doses of topical corticoids with a diagnostic of epithelial endogrowth. In the absence of response, PPK was performed and
Candida albicans was found in AP as well as in culture. The patient was treated with Natamycin eye drops and oral ketoconazol without signs of relapse although the results were of hand movement vision due to vascularization. The other receptor did not present disorders (17).Several cases have been described in DSAEK , although in that case the interface was less accessible to sampling and management was more complicated than in DALK. The case of Ortiz-Gomariz debuted at month 3 after surgery with plates in the interface. The patient was initially diagnosed as bacterial and treated as such without success. When the lens was removed,
Candida albicans
was identified as the causing agent. The infection was controlled with topical and systemic Voriconazol and cold penetrating keratoplasty was performed months later with a final VA of 20/200 (19).In 2009 two papers reporting Candida infection transmissions were published. In these cases, the germ was isolated in the donor sclerocorneal ring. Topical and systemic antifungal treatment was not enough and the cases were controlled with PK (20,21). The two Kitzmann cases involved the 2 receptors of a single donor which debuted after 5 weeks (20), while the Koenig case had to be enucleated as the PK did not produce the expected results (21). In March 2010, Chew reported a case of endophthalmitis due to Ca
ndida parapsilosis in a patient reintervened for DSAEK in which the evacuating incisions were the entry for the germ. The patient debuted 2 days later with opacities which were interpreted as epithelial growth and later as a rejection. Three months from onset, infection was suspected and Candida parapsilopsis was isolated which did not respond to treatment with Amphotericin eyedrops and oral Voriconazol. The patient exhibited signs of vitreous and posterior chamber involvement and after 6 days radical surgery was performed with PK, OIL and sac removal, anterior vitrectomy and intrachamber injection of Amphotericin and Voriconazole. Postop, intravitreal Amphotericin was injected on day 3 and oral Voriconazole 200 mg/12 hours and topical Amphotericin in descending dosage were maintained up to suspension after 2 months. At month 6, BCVA was of 20/40 cc (22).In 2011, 3 new cases were reported in DSAEK (23,24). Yamazoe presented a case with positive ring culture which 34 days after DSAEK presented infiltrates at the edge and paracentesis. The patient was initially treated with IV and topical Voriconazole and topical Mycafungin without results. Accordingly, the lenticle was removed and Mycafungin was applied intrachamber and intra-vitreous. Finally, with the cornea being opaque and vascularized, PPK was performed two months later. The graft remained clear 6 months later. Therapy was maintained with topical Voriconazole and Mycafungin (23). The latest case, recently published by Lee, involving 2 cases after DSAEK, was diagnosed with confocal microscopy as fungal interface infection. This was confirmed in a histological study of the button after PPK (24).
III. OUR EXPERIENCE WITH A CANDIDA INFECTION IN DALK
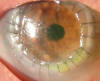
DALK was performed without complications on a 70-year-old patient with herpetic keratitis for 14 years and 2 months before had undergone treatment with autologous serum and contact lens due to persistent epithelial defect. At the time of the surgery, the eye was quiet and exhibited opacity and thinning in the inferior temporal area (fig. 1). Two months after surgery, small «keratitic precipitates» were observed which did not respond to corticoids and slowly increased in size. In the course of the 3rd month,
Candida guilliermondi was observed in a direct sample of the interface.
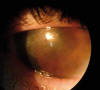
Fig. 1: Preop
appearance. Eye is quiescent, without signs of active stromal infection.
How did the fungus access the interface?
How can
Candida obtain access to such a remote place? At surgery time, either through an infected donor (the most frequent occurrence) or through the material and instruments utilized during surgery, which is unlikely. A third possibility is the one proposed in this paper, i.e., that the infection existed in the receptor as an added process to the base pathology, in this case herpetic pathology, without prior microscopic repercussions. A possible risk factor in this case could be the use of contaminated autologous serum and/or therapeutic lens which gave rise to incipient keratitis.It is nearly impossible for a fungus to penetrate non-ulcerated intact epithelium or through a properly sutured surgical wound, although it could become contaminated through an epithelial continuity solution at some time in the postop period. We had a delay in the reception of the anatomopathologic report, which became known to us when we had already isolated the
Candida, which led to a delayed diagnostic although fortunately the evolution was very slow. Otherwise, we would have found ourselves in the situation described in the literature concerning a keratoplasty which we knew could be infected by a fungus, although in a worse situation because the infection would have extended beyond the donor button.
How do we isolate the Candida from the interface? Sample taking
A simple and feasible interface sample taking technique based on slit lamp was planned. Under topical anesthesia we performed an epithelial «aperture» with an insulin needle, similar to the technique utilized to raise a flap from a Lasik to make adjustments between 2 points. It was not necessary to remove sutures, and a soft movement was applied with a 30° cannula to access the intherphase and infiltrates area, in this case at approximately 3 mm. In the first take we attempted to vacuum out an infiltrate but it was not possible because it is difficult to recover the extracted matter from the syringe for culturing. In the second take, which did allow a culturing of
Candida guilliermondii, a sweeping movement was performed without aspiration over the area to impregnate the cannula with matter. The culturing was seeded during the same procedure without visible consequences and the epithelium closed rapidly. The procedure was repeated up to three times without complications. It must be emphasized that we were fortunate to have the support of the Mycology Dept. which typified the Candida and carried out the antifungal susceptibility test (AST). A review of the literature shows that in the vast majority of cases ASTs are not available and that, even if they have interpretation-related limitations, they provide highly valuable information for selecting the best available antifungal product.After 5 days of incubation at 35°C, the Colombia Agar Blood exhibited abundant groups of under 1 mm diameter of a slow-growing yeast phenotypically and genotypically identified as
Candida Guillermondi, resistant to Fluconazole, Itraconazole and Candinas, but sensitive to Voriconazole (CIM 0.75) and Amphotericin (CIM 0.19).Three months later, as lesions were seen to be growing very slowly, a new interface sample was taken, isolating abundance groups of
C. guilliermondii showing in AST some resistance to voriconazole (CIM 2) and high sensitivity to Amphotericin (CIM 0.032). A confocal microscopy study made at this point revealed rounded structures in the interface which could be yeasts (fig. 2).

Fig. 2: Confocal
Microscopy .Round and deep refringent bodies which could be yeasts.
Donor ring culture was negative but the anatomic pathological study of the removed button reported the presence of yeast, although preop we had no clinical data on fungal infection. This confirms the importance of performing anatomopathologic studies of all removed buttons.
Prior to this case, infection by Candida Guilliermondii was reported only once, which oddly also took the clinical form of lens keratoplasty in a PPK infection. The donor culture was also negative and 8 months after surgery the patient exhibited whitish spots diagnosed as due to Candida Guilliermondii in AP when performing lamellar therapeutic keratoplasty. After recurrence at month 3, PPK was carried out with 10 months follow-up without recurrence (25).
How did clinical signs evolve?
Photographs were taken at each visit. This was very useful to detect minute changes. In two of the published cases, initially epithelial growth was considered due to the confocal microscope finding of refringent round bodies, which delayed the diagnostic (18,22). The appearance of infiltrates in the DALK interface is initially easy to erroneously identify as keratic precipitates. However, their continued growth made its location more obvious and the suspicion of infection increased. We observed said infiltrates 15 days after surgery (fig. 3A) and interpreted them as precipitates. As the patient did not respond to corticoids and the size increased we concluded they were in the interface and not on the endothelium (figs. 3B, 3C and 3D). Initially they appeared cotton-like but, after the Voriconazole treatment did not prove efficient, they “mutated” to a crystalline form (fig. 3E). Infectious crystalline keratopathy (ICK) is a chronic infection usually caused by
Streptococci viridans even though other microorganisms including Candida have been described. In the pathogenesis of ICK the formation of a biofilm is important. This biofilm is a grouping of germs within a broad exopolymer matrix (extracellular polymer) which prevents the passage of antibiotics.After intrastromal treatment infiltrates became paler. In the serial photographs we observed that during the first 10-15 days no sign of regression was seen. At that stage infiltrates began to attenuate although a cloudish appearance remained (fig. 3F).
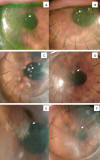
Fig. 3: Photographic
follow-up of central loci (arrow). A: Small «keratic precipitate» two weeks
post-DALK. B and C: Appearance at week 6 and 9, showing slow growth; at week 9
interface sample was taken. D: Week 12, 3 weeks after injection and beginning of
oral treatment with Voriconazole. E: at week 16, lens aspect was taken; in a new
take, resistance to Voriconazole was detected. F: after repeated intra-stromal
treatment with Amphotericin, the dots became pale, with persistence of
cloudiness.
The patient exhibited several episodes of pain and pseudo-hypopion which receded with corticoids, with aqueous culture during an acute outbreak proving negative. Initially, the cotton-like areas were central and paracentral towards temporal (area of previous herpetic involvement) and subsequently appeared in the periphery, within the sac we made with the third Melles spatula to dissect almost up to the limbus (fig. 4). Doubtlessly, this area had been under-treated with intra-stromal injections in the central area because initially we did not perform an additional peripheral injection as the stroma in the area was very thin.

Fig. 4: Area in DALK sac
at 8 o’clock.
During follow-up we discarded several times the option of performing hot PPK because we didn’t know the actual extension of the peripheral receptor cornea due to the risk of peripheral candidiasic relapse.
After the Candida areas disappeared (fig. 5) we decided to wait a few months to «cool off» the process. When the infection was regarded as under control, after three months without anti- fungal therapy and new areas, the progressive thinning and opacity prompted us to plan a tectonic PPK. The patient exhibited two central melting episodes (negative culture) which gave rise to extreme thinning. We believed this could be due to incipient herpetic necrotizing keratitis as the patient maintained base treatment with 400 mg oral aciclovir every 12 hours months before DALK up to this time.

Fig. 5: Apparently
inactive condition after treatment with intra-stromal injections.
Before scheduling the PPK we performed BMU with the aim of detecting ciliary body alterations because the eye appeared somewhat hypotonic and the echography did not show evidences of vitreous-retinal pathology. It did not reveal anomalies in the posterior chamber or the ciliary body. In the days prior to PPK an OCT-Cirrus was carried out which showed extreme inferior paracentral thinning with signs of immediate perforation (fig. 6).

Fig. 6: A: OCT Image
with imminent perforation, 48 hours before PPK. B: Cornea with central melting
and thinning.
How did we treat it?
In the light of the descriptions of the three reported cases, our initial approach was not to raise the button due to the risk of rupture and introducing the fungus into the anterior chamber. In the Fontana case, where it was decided to wash and place another button, the infiltrates relapsed in the interface in 15 days. Accordingly, washing and replacing did not seem to be the solution. Initially we utilized oral Fluconazole 200 mg/day due to the clinical suspicion of Candida infection after reviewing the literature. Subsequently, with the AST results, the treatment was modified. A 50-microgram intra-stromal Voriconazole injection was applied upon diagnostic, observing Descemet detachment and prominent inflammatory reaction within 48 hours, which receded with topical corticoids and Moxifloxacin. This could have been an exacerbated reaction to Voriconazole or a bacteria infection controlled by the antibiotic. No further Voriconazole injections were applied. In the first few months we opted for oral Voriconazole 100 mg/12 hours and topical in 1% eye drops, easily obtained in our country. However, when we identified resistance and maximum sensitivity to Amphotericin, we utilized its liposomal formulation (Ambisome®) intra-stromal 5 micrograms and 0.4% eyedrops thereof. As soon as it became available, we switched to Amphotericin Deoxylate (Fungizona®) and eye drops at 0.25% which had to be called off one month later due to toxicity which caused an inferior temporal ulceration that healed after suspension but left a permanent opacity. We applied three central Amphotericin Deoxylate 10 microgram injections at intervals of one week and one month. The central areas disappeared and after two months we treated with the same regime a peripheral area in the receptor within the surgical sac.
To carry out the injection we prepared Amphotericin in the same concentration as for intravitreal, applying 10 micrograms in 0.1 ml with a 30G syringe in the mid-deep stroma, injecting slowly because of resistance, and waited about 30 seconds before withdrawing the syringe to reduce reflux.
An unpublished fact is the production of a «pseudo big bubble» of medication (fig. 7) which diffuses from the stroma to the interface causing Descemet detachment. This occurred in the cases in which central injection was applied (4 occasions) but not in the three peripheral injections. In all cases, 24 hours later said bubble had disappeared completely.
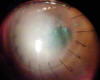
Fig. 7: Image taken
immediately after the intra-stromal injection, showing Descemet detachment.
During the PPK surgery we applied intra-chamber and intra-stromal injections of Amphotericin Deoxylate. Cultures were sterile and the button AP did not exhibit the presence of Candida. In addition, the histochemical study did not detect herpes. The postop is coursing normally one month after surgery (fig. 8).
IV. CONCLUSIONS
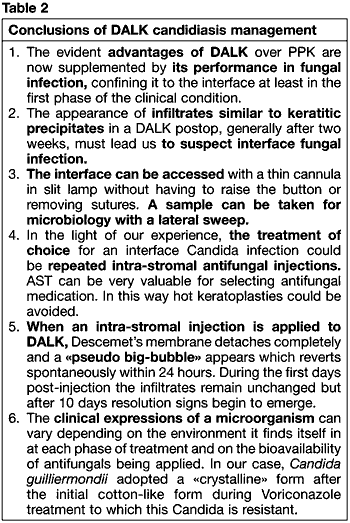
The conclusions we have drawn in our experience with this case are summarized in Table 2.
Fungal pathology in DALK is always difficult to treat due to its long evolution and frequent relapses. The combination of good sample-takiing and AST, together with local antifungal injections, could be the most convenient management of this type of pathology, reducing the need of hot and even cold penetrating keratoplasty. Many patients exhibit additional base pathologies which could be play a role such as herpetic infections which could conceal and overlap the clinical findings of the fungal condition, thus increasing the complexity of the case.
In the present case, the process was long and slow, with several treatments applied in succession which doubtlessly played a part in the need to perform a final penetrating keratoplasty; however, we believe the advanced age of the patient, with an endothelium at the preop limit and a base diagnostic of resistant herpetic keratitis, contributed to the thinning and final opacity thereof. All cultures and anatomopathological studies of the button in PPK were negative to the presence of
Candida, and therefore we think the infection has been eliminated.Our impression is that, if detected and treated at an early stage, vision could be maintained without added procedures and that, even in the presence of relevant stromal opacity, the button could be exchanged without requiring PPK.
V. REFERENCES
- Taban M, Behrens A, Newcomb RL, Nobe MY, McDonnell PJ. Incidence of acute endophthalmitis following penetrating keratoplasty: a systematic review. Arch Ophthalmol. 2005 May; 123(5): 605-9.
- Rehany U, Balut G, Lefler E, Rumelt S. The prevalence and risk factors for donor corneal button contamination and its association with ocular infection after transplantation. Cornea 2004 Oct; 23(7): 649-54.
- Keyhani K, Seedor JA, Shah MK, Terraciano AJ, Ritterband DC. The incidence of fungal keratitis and endophthalmitis following penetrating keratoplasty. Cornea 2005 Apr; 24(3): 288-91.
- Wilhelmus KR, Hassan SS. The prognostic role of donor corneoscleral rim cultures in corneal transplantation. Ophthalmology. 2007 Mar; 114(3): 440-5.
- Al-Assiri A, Al-Jastaneiah S, Al-Khalaf A, Al-Fraikh H, Wagoner MD. Late-onset donor-to-host transmission of Candida glabrata following corneal transplantation.Cornea. 2006 Jan; 25(1): 123-5.